Sodium Acetate: Preparation, Reactions and Applications
General Description
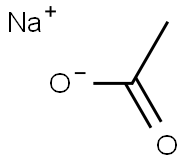
Sodium Acetate is chemically designated CH3COONa, a hygroscopic powder very soluble in water. Sodium acetate could be used as additives in food, industry, concrete manufacture, heating pads and in buffer solutions. Medically, sodium acetate is important component as an electrolyte replenisher when given intravenously. It is mainly indicated to correct sodium levels in hyponatremic patients. It can be used also in metabolic acidosis and for urine alkalinization.1

Figure 1. Properties of Sodium Acetate
Preparation
Sodium acetate is formed by the reaction of vinegar (5-8% acetic acid) with sodium carbonate (NaHCO3). In this reaction carbonic acid is formed which is further decomposed by heating produces carbon dioxide and water.2
CH3COOH + NaHCO3 → CH3COONa + H2CO3
H2CO3 → CO2 + H2O
Sodium acetate is industrially formed by the reaction of acetic acid with sodium hydroxide in an aqueous solution.
CH3COOH + NaOH → CH3COONa + H2O
Reactions
Sodium acetate is heated strongly with soda lime which is the mixture of sodium hydroxide(NaOH) and Calcium oxide (CaO) in the ratio of 3:1 by mass, methane is formed as the product.
CH3COONa + NaOH → CH4 + Na2CO3
Sodium acetate along with an alkyl halide like bromoethane can be used to form an ester.
CH3COONa + BrCH2CH3 → CH3COOCH2CH3 + NaBr
Applications
In Food
Sodium acetate may be added to food as a acidity regulator, emulsifier and preservative, seasoning, sometimes in the form of sodium diacetate. The application in ripened cheeses, processed cheese and cheese analogues, sour cream, buttermilk, yogurt, condensed milk and any dairy or whey-based drinks. Sodium acetate is also used in fruits and vegetables during processing canning or drying. You can also find the use of sodium acetate in custards, dairy-based treats like ice cream and pudding and confectionery foods like chocolate, candies and icing, vinegar, mustard, broths, soups, sauces and fat-based spreads like margarine and butter blends.3
In Pharmaceutical
Sodium acetate is used in diabetes treatment in pharmaceutical. It works as a source of sodium ions especially in cases of hyponatremic patients. Sodium has a primary role in regulating extracellular fluid volume. It controls water distribution, fluid and electrolyte balance and the osmotic pressure of body fluids. Sodium is also involved in nerve conduction, muscle contraction, acid-base balance and cell nutrient uptake.4
In Health & Personal Care Products
Sodium acetate used as buffering agent; fragrance Ingredient; masking in baby products, bath products, cleansing products, eye makeup, shaving preparations and hair and skin care products.5
In Industrial
Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling agent in chrome tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. In processing cotton for disposable cotton pads, sodium acetate is used to eliminate the buildup of static electricity.5
In Heating Pad
The most common reusable heat pads contain a supersaturated solution of sodium acetate in water. Crystallization is triggered by flexing a small flat disc of notched ferrous metal embedded in the liquid. Pressing the disc releases very tiny adhered crystals of sodium acetate into the solution which then act as nucleation sites for the crystallization of the sodium acetate into the hydrated salt (sodium acetate trihydrate, CH3COONa·3H2O). Because the liquid is supersaturated, this causes the solution to begin to crystallize over a few seconds, typically by propagating from the initial nucleation site and eventually causing the entire contained liquid to solidify, thereby releasing the thermal energy of the crystal lattice. The pad is reusable as the sodium acetate can return to the supersaturated liquid state by soaking the heating pad in boiling water and then allowing it to slowly cool to room temperature. During the process, a small amount of sodium acetate crystals will reform on the notched ferrous disk, while the rest of the sodium acetate will exist in the supersaturated liquid state, ready to be reactivated.6
References
1. Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN 978-0-19-850346-0.
2. https://byjus.com/chemistry/sodium-acetate/#Uses_of_Sodium_Acetate
3. AG, Jungbunzlauer Suisse. "Sodium Diacetate – Jungbunzlauer". www.jungbunzlauer.com
4. https://go.drugbank.com/drugs/DB09395
5. https://www.foodsweeteners.com/applications-and-uses-of-sodium-acetate-anhydrous/
6. https://home.howstuffworks.com/question290.htm
Related articles And Qustion
See also
Lastest Price from Sodium acetate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 127-09-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $6.00/kg2025-04-21
- CAS:
- 127-09-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month