Sildenafil citrate: Chemical synthesis and pharmaceutical characteristics
General description
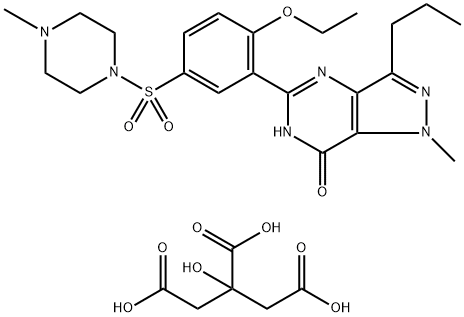
The sildenafil citrate, with the CAS No: 171599-83-0, is also known as Viagra, Sildenafil citrate. Sildenafil citrate (trade name: Viagra), an oral phosphodiesterase 5 (PDE5) inhibitor, opened a new chapter in the history of erectile dysfunction (ED) treatment. Since the United States Food and Drug Administration (FDA) approved the listing of sildenafil citrate in March 1998, great progress has been made in the study of PDE5 inhibitors represented by this drug. With the expiration of the original research patent of sildenafil, it is the goal of the synthetic workers to prepare high purity sildenafil citrate stably and efficiently and adapt to industrial production. Its appearance is as follows:

Figure 1 Appearance of sildenafil citrate.
Chemical synthesis
The sildenafil citrate can be synthesized by 3 steps according to the previous work [1]. 5-[2-hydroxy-5-(4-methylpiperazinyl-1-yl-sulphonyl)phenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (90 g) was dissolved in dichloromethane (360 ml) and added triethyl amine (41 ml) at room temperature and stirred for 10 min. The reaction mixture was cooled to 0-5°C and followed by the addition of ethyl chloroformate (24ml) over 30 min under nitrogen atmosphere. The temperature of the reaction was raised slowly to 28-30°C and maintained for 24 hrs. The reaction mixture was cooled to 0-5°C and kept it for 1 hr. The product formed was filtered, washed with dichloromethane, dried and purified from methanol (270ml) to obtain 81 g of the title compound. Purity by HPLC: 98.6%. NMR Data: 1H-NMR (300 MHz in DMSO-d6): δ 0.92 (3H, t, J=7.2), 1.17 (3H, t, J=7.2), 1.68-1.75 (2H, m), 2.16 (3H, s), 3.99 (4H, br), 2.73 (2H, t, J=7.0), 4.12-4.19 (2H, t, J=6.9), 4.15 (3H, s), 7.71 (1H, d, J = 8.7), 7.93-7.97 (1H, dd, J=8.7 & 2.1), 8.01 (1H, d, J = 2.0). 13C-NMR (75 MHz in DMSO-d6): δ 13.47, 13.80, 21.57, 27.03, 37.90, 45.72, 53.49,65.12, 124.51, 127.65, 130.14, 130.61, 132.82, 137.30, 144.96, 146.51, 151.38, 151.66, 154.36.
The product (50g) was dissolved in ethanol (150ml) in an autoclave and then added dicyclohexylcarbodimide (29.8g). The reaction temperature was raised to 110 °C with internal pressure of 1.8-4.0 kg/cm2 and maintained for 6 hours followed by cooling to room temperature. The solvent was distilled off to get the crude Sildenafil base. The base thus obtained was dissolved in dichloromethane (380ml), filtered and filtrate was distilled out completely to get solid material, which is again dissolved in a mixture dichloromethane and isopropyl ether. The crude obtained was recrystallized from ethanol (260ml) to obtain 17.4gm of pure Sildenafil base. Purity by HPLC: 99.77%.
Sildenafil base (50 g) was dissolved in acetone (850 ml) at 55°C and then slowly added citric acid solution (20 g in 100 ml acetone) over 45 min and maintain the reaction mixture for about 30 min. The reaction mixture was cooled, filtered and dried to get 65 g of Sildenafil citrate. Purity by HPLC: 99.85%.
Application
Since its approval by the US Food and Drug Administration in March 1998, sildenafil citrate has been used by millions of men for the treatment of erectile dysfunction. Sildenafil citrate, a PDE5 inhibitor with high selectivity, increases the level of cyclic guanosine monophosphate (cGMP) in the body [2]. The accumulation of cGMP then leads to a series of cellular changes that concludes with a decrease in intracellular calcium levels and the relaxation of smooth muscles. Sildenafil citrate was first approved for erectile dysfunction in 1998, but since then, additional uses for this drug have also been found. In 2005, it was approved for PH in adults and consequently an interest in research arose, questioning whether or not it could also be used successfully in infants and children [3]. Today, Sildenafil citrate is considered an encouraging treatment in PH in infants and children, although it is still used off-label with the need for further studies. Therefore, it should only be administrated under careful observation of a paediatric cardiologist [4].
Pharmacokinetics
Two-way cross-over study, 12 healthy male volunteers received a single 50 mg sildenafil dose by the sublingual and supralingual administration routes. Plasma sildenafil was determined up to 12 h post-dose. Peak concentration (C max) and area under concentration-time curve (AUC0–t) were calculated and compared between the two administration routes by analysis of variance (ANOVA) [2]. Sublingual and supralingual administration can be claimed equivalent regarding the extent of sildenafil exposure since AUC 0–t 90 % CIs corresponded to 94.90–110.58% and were within the pre-specified acceptance range. C max 90% CIs (79.92–125.57%) were only slightly outside the 80.00–125.00% limits, due to the small sample size, while the time to achieve C max did not differ between treatments (p = 0.9277). Rate of exposure of the two administration routes was therefore similar. Reported treatment-related adverse events were mild to moderate headache (33.3% of subjects) and vomiting (8.3%).
Conclusions In healthy men, sublingual and supralingual administration of sildenafil citrate resulted in a remarkably similar pharmacokinetic profile and confirmed the safety of both study treatments [5].
Absorption and metabolism
The quick absorption, between 0.5–1.5 h, together with the bioavailability for oral administration of Sildenafil citrate (38–41% in adults), makes it a great route of administration due to the good compliance that is shown in patients. Additionally, an increased absorption and bioavailability has been noted when Sildenafil citrate is administrated sublingually [6]. Furthermore, oral administration has significantly lower costs, since even though bioavailability of intravenous use is 100%, increased costs are encountered using IV administration. Sildenafil citrate is metabolized in the liver, through specific enzymes (CYP3A4 and CYP2C9), and UK-103 320 is released in circulation as the main metabolite, acquiring 50% of Sildenafil’s potency. In newborns, another important factor is the CYP3A7 enzyme, whose levels are the highest after birth and decrease simultaneously with the increase of CYP3A4 and CYP2C9 during the first week of life [7].
Toxicity and side effects
Considering Sildenafil’s mechanism of action, which decreases pulmonary arterial pressure, concerns have been raised that Sildenafil citrate could cause serious systemic hypotension and severe haemodynamic instability. Additionally, the vasodilatation induced by the blockage of PDE5 inhibitors can lead to nasal congestion, as well as flushing, and can also lead to headaches [8]. Another controversial aspect of Sildenafil citrate is its activity on the PDE6 receptors localized in the rod and cone cells of the eye, and whether or not the drug affects the normal development of the visual function of preterm neonates. However, there has been no proven evidence of a direct link between Sildenafil therapy and ocular complications, although photophobia has been observed in some subjects [8].
References
[1]Gorantla et al. Process for the production of sildenafil citrate. From PCT Int. Appl., 2007141805, 13 Dec 2007.
[2]Hemnes et al. Sildenafil, a PDE5 inhibitor, in the treatment of pulmonary hypertension. Expert Rev. Cardiovasc. Ther. 2006, 4, 293–300.
[3]Barst et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012, 125, 324–334.
[4]Herbert et al. Early Experience of Macitentan for Pulmonary Arterial Hypertension in Adult Congenital Heart Disease. Heart Lung Circ. 2017.
[5]Loprete et al. Pharmacokinetics of a Novel Sildenafil Orodispersible Film Administered by the Supralingual and the Sublingual Route to Healthy Men. Clinical Drug Investigation, 2018, 38: 765–772.
[6]Carls et al. Substantially increased sildenafil bioavailability after sublingual administration in children with congenital heart disease: Two case reports. J. Med. Case Rep. 2014, 8, 171.
[7]Lacroix et al. Expression of CYP3A in the human liver—Evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur. J. Biochem. FEBS 1997, 247, 625–634.
[8]Wardle et al. Paediatric pulmonary hypertension and sildenafil: Current practice and controversies. Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 141–147.
You may like
Related articles And Qustion
See also
Lastest Price from Sildenafil citrate manufacturers

US $0.00-0.00/KG2025-10-09
- CAS:
- 171599-83-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T

US $10.00/box2025-08-22
- CAS:
- 171599-83-0
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock