AZD 3759: Pharmacodynamics, Mechanism of action, Toxicity, Preparation
Indication
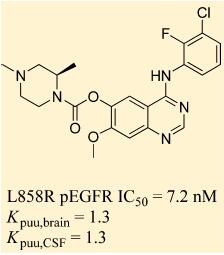
AZD 3759 is a small molecule inhibitor of the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase. It is being developed as a potential treatment for non-small cell lung cancer (NSCLC) that harbors ALK gene rearrangements. AZD3759 works by selectively targeting and inhibiting the activity of the abnormal ALK protein, which can promote the growth and survival of cancer cells in certain patients with NSCLC. Clinical trials have shown promising results for AZD3759 in this patient population, with manageable side effects reported thus far. However, further research is needed to fully evaluate its safety and efficacy. Its main design concept is to effectively cross the blood brain barrier to address the central nervous system metastasis problem of non small cell lung cancer caused by EGFR mutations[1]. Studies have shown that AZD3759 has good blood brain barrier permeability and can significantly reduce tumors. It is currently in the stage of phase I clinical research[2].

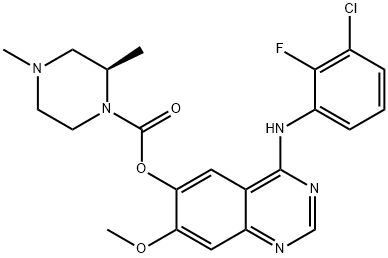
Figure 1 molecular formula of AZD 3759.
Pharmacodynamics
AZD 3759 is a novel, selective, orally active inhibitor of RORγt[3]. The differentiation and function of T-helper (Th) 17 cells are involved in the pathogenesis of autoimmune diseases such as rheumatoid arthritis, psoriasis, and multiple sclerosis. By inhibiting RORγt, AZD 3759 can suppress Th17 cell differentiation and cytokine production, leading to the suppression of inflammation and potentially providing therapeutic benefit in these autoimmune diseases. In preclinical studies, AZD 3759 has shown potent inhibition of RORγt activity, with good selectivity over other nuclear hormone receptors. It has also demonstrated efficacy in animal models of autoimmune diseases.However, it's important to note that AZD 3759 is still undergoing clinical trials, and its safety and efficacy in humans have yet to be fully established.
Mechanism of action
The mechanism of action of AZD 3759 is as a selective inhibitor of RORγt, a nuclear hormone receptor that plays a key role in the differentiation and function of T-helper (Th) 17 cells.Th17 cells are a subset of immune cells that are involved in the pathogenesis of autoimmune diseases such as rheumatoid arthritis, psoriasis, and multiple sclerosis. Th17 cells produce cytokines that promote inflammation and tissue damage in these conditions.AZD 3759 is designed to be a selective inhibitor of RORγt, meaning it does not affect other nuclear hormone receptors. This selectivity is intended to minimize off-target effects and reduce the risk of toxicity[4].
Toxicity
The toxicity profile of AZD 3759 is still being evaluated in clinical trials, and thus the full extent of its potential toxicities is not yet known. However, based on preclinical studies, some potential toxicities have been identified. In animal studies, AZD 3759 has been shown to cause reversible changes in liver enzymes at higher doses, but these changes did not result in any significant liver damage. Some decreases in white blood cell counts were also observed in preclinical studies, but this effect was considered mild and reversible. As with any new therapeutic agent, the safety and efficacy of AZD 3759 will need to be carefully monitored in human clinical trials. So far, the drug has shown an acceptable safety profile in Phase I clinical trials, with no serious adverse events reported. It's important to note that the potential side effects of AZD 3759 are likely to vary depending on the dose, duration of treatment, and individual patient factors. Patients who are considering taking AZD 3759 should discuss the potential risks and benefits with their healthcare provider[5].
Preparation
The picture 2 shows the preparation of AZD 3759[6]. It is divided into 9 steps.
Step 1:Under the protection of nitrogen at 0 ℃, add 7.3 g (93 mmol) pyridine dropwise to a mixture containing 150 mL of anhydrous dichloromethane and 9.1 g (31 mmol) of trichloromethyl carbonate (BTC), and then add 6.2 g (31 mmol) of tert butyl (3R) - 3-methylpiperazin-1-carboxylate (I) dichloromethane (60 mL) solution dropwise. Move the mixture to room temperature and stir overnight. The reaction mixture is concentrated under reduced pressure to obtain yellow solid II, which can be directly used for the next reaction without further purification. Dissolve 25.0 g (110 mmol) of 4,5-dimethoxy-2-nitrobenzoic acid (2) in 100 mL of sodium hydroxide solution (6 mol·L -1), react at 100 ℃ for 3 hours, detect the reaction by TLC, completely cool the reaction system to room temperature, pour into a mixture of concentrated hydrochloric acid and crushed ice (pH<2), filter, and vacuum dry the filter cake to obtain a yellow solid crude product (3) of 25.7 g. Under hydrogen atmosphere and room temperature, a mixture of 25.0 g (117 mmol) of compound 3 and 2.5 g of 10% mass fraction Pd-C. and 60 mL of methanol was stirred for 4 h. The reaction was completely detected by TLC. Filter, wash with methanol, combine methanol extracts, evaporate under reduced pressure to remove solvent, and obtain 14.1 g of black solid (4) in a yield of 68%.
Step 2: To a mixture of 14.0 g (76 mmol) of compound 4 and 200 ml of 2-methoxyethanol, 6.7 g (153 mmol) of formamidine was added, heated to 125 ℃, and reacted overnight. TLC detected that the reaction was complete. The reaction solution was cooled and subjected to reduced pressure distillation. The residue was poured into 250 ml of water, dropwise added ammonia to adjust the pH to 7, filtered, washed with water, and vacuum dried to obtain 10.0 g of brown solid (6), with a yield of 6%.
Step 3: To a mixture of 10 g (52 mmol) of compound 5 and 50 mL of acetic anhydride, 8.2 g (104 mmol) of pyridine was added, heated to 80 ℃, and reacted for 1 h. TLC detection showed that the reaction was complete. The reaction solution was cooled and subjected to reduced pressure distillation. The residue was poured into 200 ml of water, filtered, and the filter cake was vacuum dried to obtain 12.1 g of gray solid (6), with a yield of 99%.
Step 4: To a mixture of 12 g (51 mmol) of compound 6 and 100 mL1,2-dichloroethane, 100 ml of dichlorosulfoxide was added dropwise, followed by 10 drops of N, N-dimethylformamide. After dropping, the mixture was refluxed at 80 ℃, the solvent was evaporated under reduced pressure, and the residue was poured into 150 mL of water. Under an ice bath, a saturated aqueous sodium bicarbonate solution was slowly added to the residue, the pH was adjusted to 8.0 to 9.0, and then filtered. The filter cake was washed with water, and vacuum dried to obtain 12.1 g of white body (7), The yield was 93.7%. The purity is 97.68%.
Step 5: To a suspension of 10 g (40 mmol) of acetonitrile (100 mL) of compound 7, 6.3 g (43 mmol) of 2-fluoro-3-chloroaniline was added, and the mixture was refluxed overnight at 80 ° C. The solvent was removed by vacuum distillation to obtain 14.3 g of white solid (8) in a yield of 89%.
Step 6: To a mixture of 14.3 g (40 mmol) of compound 8 and 150 mL of methanol, 13.7 g (10 mmol) of anhydrous potassium carbonate was added, and the mixture was stirred overnight at room temperature. The solvent was evaporated under reduced pressure, the residue was poured into 300 ml of water, filtered, and the filter cake was vacuum dried to obtain 9.8 g of white solid (9) in a yield of 7.5%.
Step 7: To a mixture of 9.8 g (31 mmol) of the complex 9 and the prepared intermediate II, NNA-dimethylmethanolamine 6 (150 mL), 85 g of 62mo anhydrous potassium citrate was added at room temperature, and the reaction was complete by stirring overnight for TLC testing. The mixture was vacuum dried into 150 mL of ice water to obtain 15.4 g of yellow solid (10) in a yield of 92%.
Step 8: Dissolve 15.0 g (2 mmol) of compound 10 in a mixed solution of 160 mL of dichloromethane and 40 mL of trifluoroacetic acid, and react at room temperature for 1 hour. The reaction was completely detected by TLC. Add 150 mL of water to the residue by vacuum evaporation and adjust the pH value to 8.0~9.0 with saturated sodium hydrogen borate aqueous solution. Extract the mixture with 10 ml of dichloromethane and combine the organic layer with anhydrous sodium carbonate to dry and vacuum evaporate the solvent to obtain a yellow solid (11) 114g, The yield was 93%.
Step 9: To a solution of 11.0 g (24.6 mmol) of dichloromethane (110 mL) of compound 11, 1.2 g (37 mmol) of paraformaldehyde was added, and the reaction was stirred under nitrogen atmosphere at room temperature for 0.5 h. To the reaction system, add 6.2 g (98.4 mmol) of sodium cyanide borohydride and 100 mL of acetic acid in methanol (40 ml) solution, stir at room temperature for 2 hours, add 100 mL of water to the reaction system, adjust the pH to 8.0~9.0 with a saturated aqueous solution of sodium bicarbonate, separate the solution, extract with dichloromethane, merge the organic layers, dry with anhydrous sodium sulfate, evaporate under reduced pressure to remove the solvent, and separate and purify to obtain 10.0 g of white solid (1), with a yield of 88% and a purity of 96.05%.
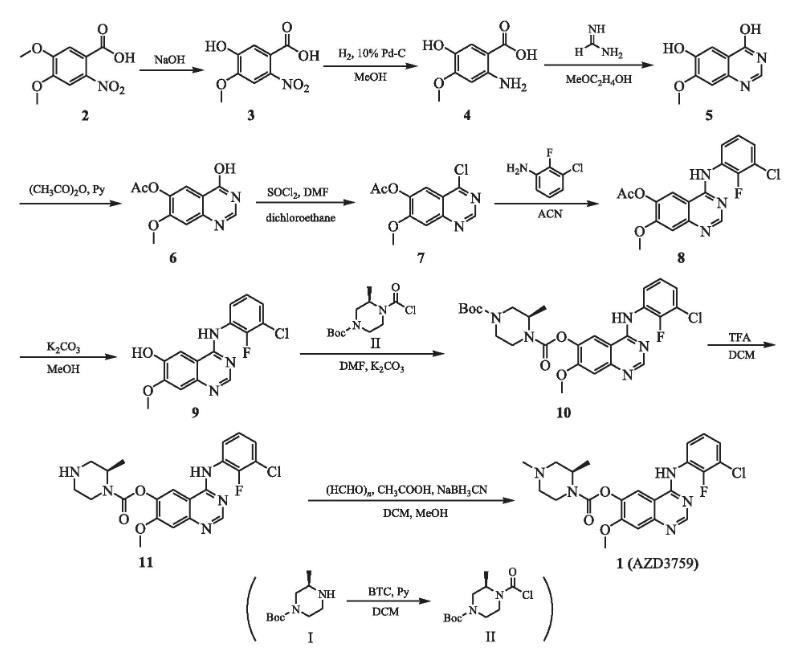
Figure 2 Preparation of AZD 3759.
References
[1] Zeng Q B,Wang J B,Cheng Z Q, et al. Discovery and evaluation of clinical candidate AZD 3759, apotent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor(EGFR TKI)[J].J Med Chem,2015,58(20):8200-8215.
[2] Ahn M J, Kim D W, Cho B C,et al. Activity and safety of AZD 3759 in EGFR-mutant non-small-cell lung cancerwith CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study[J].Lancet Respir Med,2017,5(11):891-902.
[3] Wu Qingjun,Hua Ailian,Sun Yaoguang,Ma Chao,Tian Wenxin,Huang Chuan,Yu Hanbo,Jiao Peng,Wang Shuanghu,Tong Hongfeng,Qiu Weiwen. Determination and pharmacokinetic study of AZD-3759 in rat plasma by ultra performance liquid chromatography with triple quadrupole mass spectrometer.[J]. Thoracic cancer,2018,9(11).
[4] Attwa Mohamed W,AlShakliah Nasser S,AlRabiah Haitham,Kadi Adnan A,Abdelhameed Ali S. Estimation of zorifertinib metabolic stability in human liver microsomes using LC-MS/MS.[J]. Journal of pharmaceutical and biomedical analysis,2022,211.
[5] AlShakliah Nasser S,Kadi Adnan A,Aljohar Haya I,AlRabiah Haitham,Attwa Mohamed W. Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry.[J]. RSC advances,2022,12(32).
[6] Zhang Yue, Wei Huating, Gao Yueying, Tang Chunlei. Improvement of the synthesis process of EGFR inhibitor AZD3759 [J]. Chinese Journal of Pharmaceutical Chemistry, 2020,30 (04): 223-227.
Related articles And Qustion
See also
Lastest Price from AZD 3759 manufacturers

US $0.00/kg2025-03-07
- CAS:
- 1626387-80-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons

US $0.00/g2025-01-13
- CAS:
- 1626387-80-1
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month