Practical Applications of Sodium Acetate
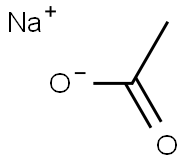
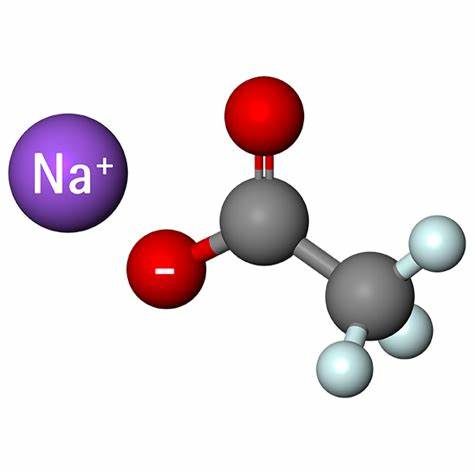
Sodium acetate salt, or simply sodium acetate, has many practical uses. It is the conjugate base of a weak acid, meaning that it only partially ionizes when dissolved in water. This provides sodium acetate with buffering properties, that is the ability to maintain solutions at a relatively constant pH despite acid or base challenges. This property along with its low toxicity, helps explain why sodium acetate can be found in industries ranging from petroleum production to food flavoring.

Physical Properties
Physically, sodium acetate appears as a white hygroscopic or water-attracting crystalline powder. The pure substance has a melting point of 58 degrees C or 136 degrees F, and completely decomposes at the boiling point of 120 degrees C or 248 degrees F. Sodium acetate dissolves readily in water, having a solubility of 500 g/L at 20 degrees C. Crystals have a basic pH of about 7.5 to 9.0.
Safety
The safety of sodium acetate has been studied extensively in rat and mouse animal models. When given orally, the lethal dose that will kill half a population of rats is 3530mg of sodium acetate per kg of rat body weight. If inhaled rather than ingested, the dose required to kill half the rat population is much higher, over 30 g/m3 per hour.
In mice, a subcutaneous or under the skin injection of 3200mg/kg of body weight will kill half of a mouse population, similar to the ingestion of sodium acetate in rats. However, orally mice can withstand much more than rats; the lethal dose for half the population of mice being 6891mg/kg of body weight. In humans, inhalation of sodium acetate may cause a cough and sore throat. Direct skin or eye contact may cause redness and irritation. However, overall, toxicity in humans is minimal.
Practical Applications
Sodium acetate finds use in an extremely diverse range of industries. In the textile industry, sodium acetate neutralizes sulfuric acid waste streams and improves the wearing quality of finished fabrics. In photography, sodium acetate constitutes part of the developer solution and acts as a photo resist agent. In rubber production, sodium acetate retards vulcanization helping control the overall process.
Sodium acetate added to foods acts as a preservative, and a flavoring agent. In particular, potato chips with sodium acetate have a distinctive "salt and vinegar" taste. Sodium acetate and acetic acid solutions act as buffers to maintain relatively constant pH, a property useful both for biochemical research reactions, the petroleum industry and in the cosmetic industry. In the medical field, sodium acetate solutions treat patients with high blood acid levels and/or low sodium levels.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium acetate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 127-09-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $6.00/kg2025-04-21
- CAS:
- 127-09-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month