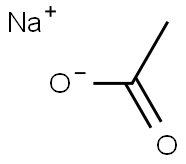
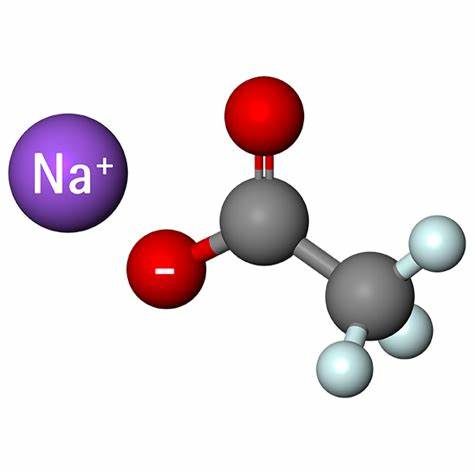
Is Sodium acetate an acid or a base salt?
Sodium acetate (CH3COONa) is in some cases a basic salt. CH3COONa is produced by the reaction of acetic acid with sodium hydroxide and it is the salt of the acetate ion. According to Lowry -Bronsted acid base theory, an acid is a substance that tends to give protons to any other substance, while a base is defined as a substance that tends to receive protons from other substances. Acids and bases interact to produce an ionic compound known as a salt. Whereas acetic acid has a dissociation constant equal to 1.8 × 10-5 and is a weak acid, sodium hydroxide is a strong base. Acids have conjugate bases. The weak base acetic acid loses its proton to form the conjugate base acetate ion. This is a stronger base. It is a conjugate base of a weak acid. It is a stronger base. Hence, we can say that sodium acetate is a basic salt.
You may like
Related articles And Qustion
Lastest Price from Sodium acetate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 127-09-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $6.00/kg2025-04-21
- CAS:
- 127-09-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month