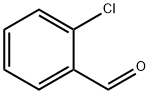
2-Chlorobenzaldehyde: The synthesis and application
General description
2-Chlorobenzaldehyde is used as medicine and dye intermediate. It is proved as a novel analytical chelating reagent to developa simple, fast and selective liquid–liquid extractive spectrophotometric method for trace level determination of copper(II). The pesticide Mithijing produced with o-chlorobenzaldehyde can control mites on dry crops and fruit trees. O-chlorobenzoxime can be obtained by oximation of o-chlorobenzaldehyde, and o-chlorobenzoxime can be obtained by further chlorination, both of which are pharmaceutical intermediates. Its appearance is as follows:

Figure 1 Appearance of 2-Chlorobenzaldehyde.
Chemical synthesis
The 2-Chlorobenzaldehyde can be synthesized by single step according to the previous work [1]. A solution of BTC (0.41 g, 1.39 mmol) in dry CH2Cl2(5 mL) was cooled in an ice-salt bath under an atmosphere of N2. A solution of I (1.24 g, 4.17 mmol) in dry CH2Cl2(5 mL) was added dropwise for 0.5 h, at -15 °C. Stirring was continued for 0.5 h, and a solution of benzyl alcohol (0.3 g, 2.78 mmol) in dry CH2Cl2(5 mL) was added dropwise for 0.5 h, at -15 °C. After stirring for 0.5 h, Et3N (0.84 g, 8.34 mmol) was added slowly while the temperature should be controlled below -15 °C. When the reaction was completed, 10% HCl solution in water was added dropwise until the pH of the reaction solution reached 2 under ice bath. The mixture was extracted with n-hexane or petroleum ether (10 mL× 2), decanted. The product was acquired after organic layer was concentrated and purified by flash chromatography (SiO2; n-hexane). The water layer was used for the recovery of 2-Cholorbenzaldehyde. 2-Cholorbenzaldehyde: (326 mg, 92.7% yield); liquid (commercially avaiable, liquid). 1H NMR (400 MHz, CDCl3): δ 10.48 (s, 1H), 7.92 (dd, J1 7.6 Hz, J2 1.6 Hz, 1H), 7.56-7.50 (m, 1H), 7.46 (dd, J1 8.0 Hz, J2 1.2 Hz, 1H), 7.42-7.37 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 189.6, 137.9, 135.1, 133.3, 130.6, 129.4, 127.3.
Application
2-Chlorobenzaldehide has many applications [2] and is the intermediate of indole ester, a plant growth regulator. It can be used as a dye, pesticide and pharmaceutical intermediate, as a raw material for the synthesis of acaricide tetrazine, and can also be used for the synthesis of another new acaricide, flutenzine. It is also used to manufacture o-chlorobenzoyl chloride, which is the main raw material of medical drugs such as o-chlorobenzoyl chloride and cloxacillin sodium.
Pharmacokinetics
2-Chlorobenzaldehyde might be produced when a moist skin is exposed to the riot control agent CS. CS-hydrolysis to 2-chlorobenzaldehyde and malononitrile occurs both in vitro and in vivo [3]. Percutaneous absorption, biotransformation and elimination of 14C labelled 2-chlorobenzaldehyde was investigated in the rat [4]. Following IV (25 uL/kg) and IP (37.5 uL /kg) .14C 2-chlorobenzaldehyde administration to rats, the plasma radioactivity declined rapidly over a 24 h period with similar plasma radioactivity-time profiles. Following cutaneous administration (75 uL/kg) in a closed glass-cup on the skin a slow skin penetration occurred as indicated by plasma radioactivity levels. A slow increase in plasma radioactivity was followed by a slow decline of radioactivity in plasma over a 3-day period. Most of the radioactivity was found in the urine with low levels in faces and exhaled air. The cutaneous administered radioactivity was also partly recovered from the glass-cup.
For the qualitative and quantitative determination of metabolites in urine, a thin layer chromatography-radioautography method was' used. The metabolic patterns of urinary excreted metabolites following cutaneous application and systemic administration of 14C 2-chlorobenzaldehyde to rats were very similar. No parent compound was recovered from the rat urine. 2-Chlorohippuric acid was the principal urinary metabolite. Quantitatively, the urinary excretion of. 14C 2-chlorobenzyl alcohol following cutaneous application differed substantially from that after the systemic administration. There was no evidence of storage in the skin or skin toxicity of 2-chlorobenzaldehyde following cutaneous application [4].
References
[1]Ye et al. Efficient and mild Swern oxidation using a new sulfoxide and bis(trichloromethyl)carbonate. Synthetic Communications, 2016, 46(10), 885-892.
[2]Schafer et al. Electron-Diffraction Study of Molecular-Structure of 2-Chlorobenzaldehyde. Journal of Molecular Structure. 1976, 31(1) , 29-37.
[3]Kluwe et al. Encephalopathy in rats and nephropathy in rats and mice after subchronic oral exposure to benzaldehyde. Fd, Chern. Toxic., 1983, 21, 245-250.
[4]Rietveld et al. Percutaneous absorption of 14C labelled 2-chlorobenzaldehyde in rats. Metabolism and toxicokinetics. 1987, 13(4), 231-240.
Related articles And Qustion
See also
Lastest Price from 2-Chlorobenzaldehyde manufacturers

US $1.00/g2025-04-21
- CAS:
- 89-98-5
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 89-98-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt