Oleamide: Physiological effects
General description
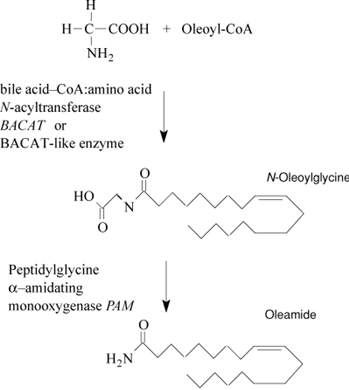
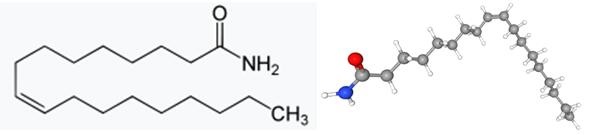
Oleamide is an amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is from N-oleoylglycine.[1] In terms of natural occurrence, oleamide was first found in human plasma. It was later shown to accumulate in the cerebrospinal fluid during sleep deprivation and induces sleep in animals.[1] It has been considered a potential treatment for mood, sleep disorders, and cannabinoid-regulated depression. In terms of its sleep-inducing effects, oleamide interacts with multiple neurotransmitter systems. Synthetic oleamide has a variety of industrial uses as a slip agent, a lubricant, and a corrosion inhibitor. Oleamide was found to be leaking out of polypropylene plastics in laboratory experiments, affecting experimental results. Since polypropylene is used in food containers such as those for yogurt, the problem is being studied. Oleamide is "one of the most frequent non-cannabinoid ingredients associated with Spice products." Analysis of 44 products of synthetic cannabinoids revealed oleamide in 7 of the products tested. Its appearance is as follows:

Figure 1 Appearance of Oleamide
Physiological effects
Oleamide demonstrates a variety of physiological effects. For example, after intraperitoneal (i.p.) administration to rats, it induces sleep and hypomotility and exhibits long-lasting hypothermic effects [2]. These effects are similar to those evoked by the activation of cannabinoid receptor systems; indeed the characteristic “tetrad” of effects evoked by cannabinoid receptor agonists in vivo is hypothermia, hypoactivity, analgesia, and catalepsy. Additionally, i.p. injections of oleamide increase food intake for up to 3 h [3], and increased appetite has long been known to be a feature of cannabinoid pharmacology. This is the basis of the very recent clinical introduction of the cannabinoid antagonist rimonabant (SR 141716A) as an antiobesity agent. Federova and coworkers (2001) reported that, in addition to inducing hypomotility and hypothermia, oleamide has antianxiety and analgesic effects. The basis of these actions of oleamide appears to involve a variety of neurotransmitter systems other than those for endocannabinoids including GABA, dopamine, and 5-hydroxytryptamine (5-HT). For example, the effects on hypomotility in rats are resistant to the effects of rimonabant but can be blocked by the dopamine D 2 receptor antagonist, L 741626, whereas bicuculline, an antagonist at GABA A receptors reduced the analgesia and hypothermia [4].
The literature concerning the interactions of oleamide with cannabinoid receptors is conflicting. Early reports indicated that oleamide did not interact appreciably with or activate cannabinoid receptors and this led to the suggestion that its cannabinoid-like actions might be due to an “entourage effect”. Many articles in the literature concern the interactions of oleamide with gap junctions, which are also sensitive to anandamide, in particular as a tool to inhibit their function. In view of the importance of gap junctions in the cardiovascular system, in the heart, endothelial cells, and vascular smooth muscle, this aspect of its biology is of particular relevance. Anandamide mediates some of its actions through activation of the vanilloid receptor TRPV1, but in behavioral work, it was reported that pretreatment of rats with capsazepine, a vanilloid receptor antagonist, did not affect the responses to oleamide [4]. An additional postulated site of action for oleamide is the voltage-gated Na + channel [5]. Oleamide inhibited the binding of [3H]batrachotoxinin A to mouse brain synaptosomes with an IC 50 of 39.5 µM and, at a concentration of 10 µM, it decreased peak Na + currents in cultured N1E115 neuroblastoma cells in a voltage-dependent manner.The concept of the “entourage” effect was first put forward with regard to the endocannabinoid, 2-AG. It arises from the observations that 2-AG is commonly released with congeners, which are inactive at cannabinoid receptors but are substrates for FAAH.The early observations that oleamide did not appear to directly interact with cannabinoid receptors, but raised concentrations of the endogenous CB 1 receptor agonist anandamide, led to extension of the idea to oleamide.
References
[1]McKinney, Michele K.; Cravatt, Benjamin F. (June 2005). "Structure and Function of Fatty Acid Amide Hydrolase". Annual Review of Biochemistry. 74 (1): 411–432. doi:10.1146/annurev.biochem.74.082803.133450. PMID 15952893.
[2]Huitron-Resendiz S, Gombart L, Cravatt BF, Henriksen SJ (2001) Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp Neurol 172:235-243.
[3]Martinez-Gonzalez D, Bonilla-Jaime H, Morales-Otal A, Henriksen SJ, Velazquez-Moctezuma J, Prospero-Garcia O (2004) Oleamide and anandamide effects on food intake and sexual behavior of rats. Neurosci Lett 364: 1-6.
[4]Fedorova I, Hashimoto A, Fecik RA, Hedrick MP, Hanus LO, Boger DL, Rice KC, Basile AS (2001) Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J Pharmacol Exp Ther 299:332-342.
[5]Nicholson RA, Zheng J, Ganellin CR, Verdon B, Lees G (2001) Anesthetic-like interaction of the sleepinducing lipid oleamide with voltage-gated sodium channels in mammalian brain. Anesthesiology 94:120-128.
You may like
Related articles And Qustion
See also
Lastest Price from Oleamide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt

US $6.00/kg2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month