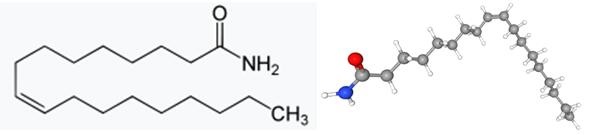
Discovery and Biosynthesis of Oleamide
Discovery
In the mid-1990s, a novel lipid agent was identified in the cerebrospinal fluid of cats deprived of sleep. It was first thought to be a diene, and named cerebrodiene, before it was subsequently identified as oleamide, the cis-isomer of an amide of a monounsaturated 18-carbon fatty acid. The amount of oleamide increased by 3- to 4-fold in the cerebrospinal fluid of rats deprived of sleep for 6 h or more and was thought to play a significant role in sleep regulation.
Not only is oleamide found endogenously, it can be formed de novo in brain microsomes using oleic acid and ammonia as substrates; this is probably an enzymatic reaction, since the synthesis was absent in boiled preparations. Use of fatty acid amide hydrolase (FAAH, the enzyme usually responsible for the degradation of FAAs) inhibitors prevented synthesis of oleamide from ammonia and oleic acid in cultured mouse neuroblastoma N18TG2 cells. However, these might not represent the usual starting materials, and the quantities of ammonia required are high. Therefore, the primary enzymatic route for oleamide biosynthesis has yet to be elucidated. There is a consensus that the process first involves oleic acid being converted to oleoyl-CoA and N-oleoylglycine. Oxidative cleavage by peptidylglycine α−amidating monooxygenase (PAM), a copper-containing enzyme, then follows with the resulting formation of oleamide.
Biosynthesis
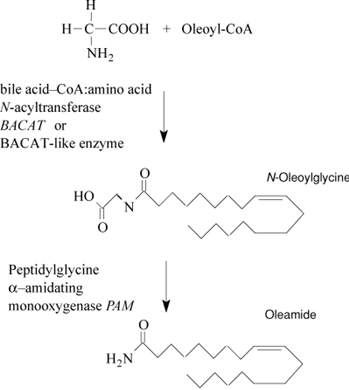
The synthetic pathway for oleamide that is usually proposed involves production of N-oleoylglycine, possibly simply from oleoyl-CoA and glycine, and then production of oleamide by PAM. The enzyme producing the oleoylglycine has yet to be identified, but BACAT, or a related enzyme, has been suggested as a possible candidate. Oleamide is inactivated by hydrolysis with FAAH, but this enzyme can also synthesize oleamide from oleic acid and ammonia and this might also provide a synthetic route to oleamide, although high concentrations of ammonia are required in vitro.
Many C-terminally amidated neuropeptides, such as substance P, follow a similar biosynthetic route in vascular cells, and PAM has been shown to be able to act upon a wide range of substrates and to require minimally an acylglycine moiety to allow oxidative amidation. PAM activity can be inhibited reversibly by [(4-methoxybenzoyl)oxy]acetic acid and irreversibly by 4-phenyl-3-butenoic acid (PBA), which functions as a mechanism-based inhibitor. PBA has been used to inhibit the production of substance P from its extended precursor, glycyl substance P, in cultured bovine aortic endothelial cells, and this inhibitor might, therefore, be a useful agent for inhibiting the production of oleamide in the heart and circulation, should it occur. Interestingly, recent evidence has been put forward showing that oleoylglycine, the putative precursor for oleamide, has biological effects independent of its conversion to oleamide. For example, infusion of oleoylglycine had no effect on plasma levels of oleamide, although it induced hypothermia in rats with a maximum effect about half that of the same dose of oleamide (80 mg/kg). Disulfiram, a copper-chelating agent that can be used to inhibit PAM, had very similar effects on the hypothermic response; it was therefore suggested that this agent was acting downstream of oleamide production and that this would limit its usefulness in studies of oleamide synthesis.
Reference
[1] C. ROBIN HILEY Pui M H. Oleamide: A Fatty Acid Amide Signaling Molecule in the Cardiovascular System?[J]. Cardiovascular Therapeutics, 2007, 25 1: 46-60. DOI:10.1111/j.1527-3466.2007.00004.x.
You may like
Related articles And Qustion
See also
Lastest Price from Oleamide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt

US $6.00/kg2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month