Oleamide: natural occurrence, biologic actions and biosynthesis
General Description
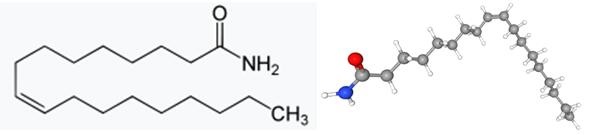
Oleamide is a long-chain primary fatty acid amide that is naturally found in various biological fluids, including human serum, rat cerebral spinal fluid, and human breast cancer lines. It has also been detected in human tear film, although the extent of its contribution to nonpolar lipids in this secretion is still unclear. Oleamide interacts with multiple receptor systems, such as GABAA and serotonin receptors, and its effects are sensitive to its structural features. It has been implicated in sleep regulation, depression, anxiety, and analgesia. Oleamide inhibits gap junction communication in various cell types, and its biosynthesis may occur through different mechanisms, including the conversion of oleoylglycine and the condensation of oleoyl-CoA and ammonia. Further research is needed to fully understand the biologic actions and biosynthesis of oleamide.

Figure 1. Oleamide
Natural occurrence
Oleamide is a long-chain primary fatty acid amide that has been found in various biological fluids including human serum, rat cerebral spinal fluid, and human breast cancer lines. The first quantitative report on oleamide levels in rat plasma and cerebral spinal fluid showed concentrations of 10 ng/mL and 44 ng/mL, respectively. Oleamide has also been detected in human tear film but the relative contribution to nonpolar lipids in this secretion is unclear due to discrepancies in reported findings. It is important to note that oleamide is widely used as a slip agent in the production of polyethylene products commonly used in laboratory research, which may complicate the interpretation of its natural occurrence. Biosynthetic labeling approaches using radioactive or stable mass isotopes have been employed to demonstrate oleamide biosynthesis by different cell types. Recent studies have shown that oleamide synthesis is not restricted to a single cell type and may be a process shared by many, if not all, cells. 1
Biologic actions
Oleamide, a compound found in the human body, has been shown to interact with several receptor systems, such as GABAA, serotonin2A, serotonin2C, and serotonin7 receptors. Although a specific receptor for oleamide has not been identified, it is believed that oleamide exerts its effects through allosteric interactions with these receptor proteins. The effects of oleamide on these systems are highly sensitive to its structural features, including acyl chain length, double bond position, and stereochemistry. These interactions suggest that oleamide may play a role not only in sleep regulation but also in conditions like depression, anxiety, and analgesia, which involve altered serotonergic neurotransmission. Additionally, oleamide has been found to inhibit gap junction communication, which are protein channels that allow for direct electrical coupling and molecule exchange between adjacent cells. This effect has been observed in various cell types, including glial cells, neurons, epithelial cells, endothelial cells, and more. However, it is still not clear whether this cellular effect is mediated by specific oleamide receptors or through interactions with the gap junction proteins themselves. The widespread expression of oleamide-sensitive gap junctions suggests that the biosynthesis of oleamide may occur in many cell types and tissues. Oleamide is commonly used as an experimental tool for studying gap junction function. Overall, the biologic actions of oleamide involve its interactions with various receptor systems and its inhibition of gap junction communication in different cell types. Further research is needed to fully understand the mechanisms underlying these actions. 2
Biosynthesis
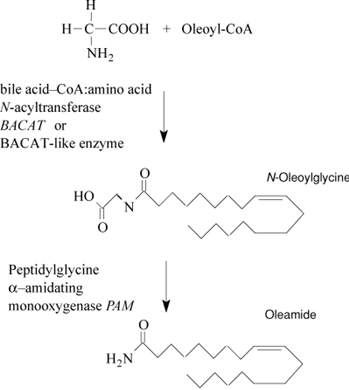
The biosynthesis of oleamide has been a focus of investigation since the mid-1990s, and several mechanisms have emerged as possibilities. The most compelling evidence supports the mechanism involving the conversion of oleoylglycine to oleamide by the neuropeptide processing enzyme, PAM. A second mechanism involving the condensation of oleoyl coenzyme A (oleoyl-CoA) and ammonia by cytochrome c has also emerged as an alternative pathway for the biosynthesis of oleamide. Other possible mechanisms for oleamide biosynthesis have been proposed, but experimental evidence supporting their biologic relevance is lacking. Recently, a circulating oleamide synthase has been discovered that is biochemically and functionally distinct from both cytochrome c and PAM. This activity is enriched in fetal bovine serum used to supplement cell culture media and is negatively charged and approximately 65,000 Da in mass. Overall, the biosynthesis of oleamide may occur through multiple mechanisms in a cell and tissue-specific manner, but further research is needed to fully understand these processes. 3
Reference
1. Arafat, E. S., Trimble, J. W., Andersen, R. N., Dass, C., and Desiderio, D. M. (1989). Identification of fatty acid amides in human plasma. Life Sci. 45, 1679–1687.
2. Laposky, A. D., Homanics, G. E., Basile, A., and Mendelson, W. B. (2001). Deletion of the GABA(A) receptor beta 3 subunit eliminates the hypnotic actions of oleamide in mice. Neuroreport 12, 4143–4147.
3. Bisogno, T., Sepe, N., De Petrocellis, L., Mechoulam, R., and Di Marzo, V. (1997). The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem. Biophys. Res. Commun. 239, 473–479.
Related articles And Qustion
See also
Lastest Price from Oleamide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt

US $6.00/kg2025-04-21
- CAS:
- 301-02-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month