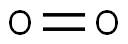How to determine the polarity of oxygen?
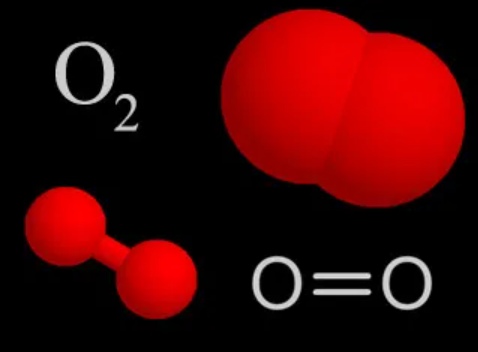
Oxygen is a chemical element with an atomic number 8 and symbol O. It lies in group 16 (chalcogen group) of the periodic table and is a highly reactive non-metallic substance. It is an oxidizing agent that forms oxides with multiple compounds readily. There are many known allotropes of oxygen, out of which O2 is the most stable one. It is also known as diatomic oxygen or molecular oxygen and has a significant presence in the atmosphere.
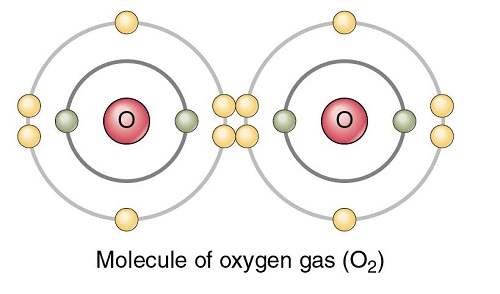
Oxygen polarity
So, is O2 Polar or Non-Polar?
The oxygen (O2) molecule is nonpolar because the molecule is diatomic and both atoms have equal electronegativity. As a result, both atoms share equal charges and there are no partial charges on any atom. Consequently, O2 comes out to be a nonpolar molecule with a zero dipole moment.
Why is O2 nonpolar?
The O2 molecule considered a nonpolar molecule due to the below parameters.
Electronegativity Difference: The electronegativity of a single oxygen atom (O) is 3.44.
When two of these atoms organize themselves with a double bond between them, it forms the structure of molecular O2 (diatomic oxygen). Since both the atoms are the same, the difference in their electronegativity is 0.
Dipole Moment of O2: Both the atoms forming O2 are the same and hence have an equal and opposite effect on each other.
The magnitude of the pull exerted on the shared electrons is equal from both sides, thereby leading to a net-zero force.
So, no charge build-up occurs at any pole and the net dipole moment of the molecular oxygen remains 0 Debye.
Symmetrical shape: The O2 molecule is linear in shape due to the diatomic molecule. Two atoms form double bonds to complete their octet and form a linear geometrical structure.