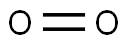Oxygen:Sources,electronic configuration,uses,production
Oxygen is the most abundant chemical element by mass in the Earth's biosphere, air, sea, and land (Box 3.8). Oxygen constitutes 49.2% of the Earth's crust by mass as part of oxide compounds such as silicon dioxide, and SiO2, and is the most abundant element by mass in the Earth's crust.
Sources
Oxygen (besides H) is the most common element in minerals and forms the basis of the anionic groups (carbonates, nitrates, borates, sulfates, chromates, phosphates, arsenates, vanadates, molybdates, tungstates, silicates, etc.). The chemically simplest minerals are found in the oxide/hydroxide class, for example, anatase (TiO2), bixbyite (Mn2O3), bo¨hmite (AlOOH), brookite (TiO2), etc.

Fig.quartz
Chemistry
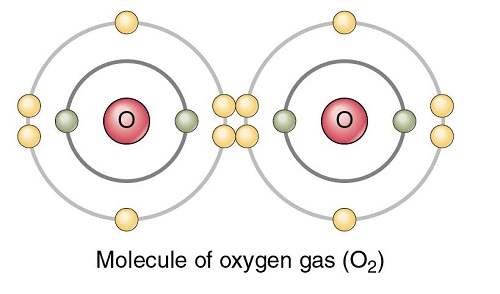
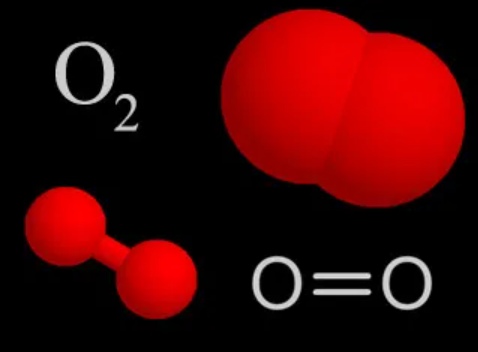
Oxygen is a member of the chalcogen group on the periodic table. The electronic configuration of the free O atom is [He]2s22p2x2p1y2p1z. Oxygen is normally divalent, though, other oxidation states such as 1/2, 0, in isolable compounds are also known. It has a very high electronegativity of 3.5, which is exceeded.
Production
Oxygen gas can also be produced through the electrolysis of water into molecular oxygen and hydrogen. DC electricity must be used: if AC is used, the gases in each limb consist of hydrogen and oxygen in the explosive ratio of 2:1.
Uses
Oxygen is used in cellular respiration and many important groups of organic molecules in living organisms including humans contain oxygen, for example, proteins, nucleic acids, carbohydrates, and fats, as do the most important constituent inorganic compounds of animal shells, teeth, and bone (calcite, aragonite, apatite, etc.). Most of the mass of living organisms contains oxygen as a part of water, H2O.