Cefazolin Sodium Salt: First-Generation Cephalosporin for Bone, Joint, and CNS Infections
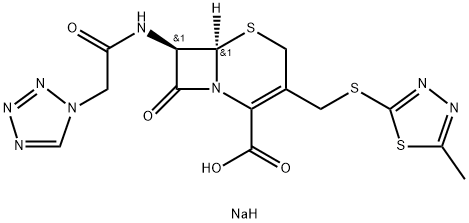
Cefazolin sodium salt is a cephalosporin organic sodium salt having [(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl and (1H-tetrazol-1-ylacetyl)amino side-groups. It contains a cefazolin(1-).Cefazolin sodium salt is a beta-lactam antibiotic and first-generation cephalosporin with bactericidal activity. Cefazolin binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity, which results in the weakening of the bacterial cell wall and cell lysis.

Continuous Cefazolin Infusion to Treat Bone and Joint Infections
Cefazolin sodium salt is a semisynthetic cephalosporin with good in vitro activity against methicillin-susceptible staphylococci (MIC90, 1 mg/liter) and nonenterococcal streptococci (MIC90s, 0.1 to 2 mg/liter) and with excellent tolerance and good bone diffusion. Several authors recommend the use of Cefazolin sodium salt for the treatment of bone and joint infections, particularly those due to Staphylococcus aureus , although only one study of this subject has been published to date. The continuous intravenous administration of β-lactams can be an advantageous way to deliver these drugs, as their efficacy is time dependent; i.e., it increases with the time that the concentrations in serum exceed the MIC for the target pathogen. When a molecule like Cefazolin sodium salt is stable over 24 h, the use of continuous infusion avoids the need for repeated intermittent injections, as the delivery device is refilled once or twice daily, which is particularly pertinent for parenteral antibiotic therapy on an outpatient basis. The treatment of chronic bone and joint infections remains difficult. It requires a multidisciplinary approach that combines the identification of the responsible pathogen(s), surgical intervention, and prolonged antibiotic therapy.[1]
Cefazolin sodium salt has been used for >30 years to treat bone and joint infections, because of its good activity against gram-positive cocci, especially methicillin-susceptible staphylococci; its excellent tolerance; its low cost; and its limited antimicrobial spectrum. No drug interaction has been observed. Because Cefazolin sodium salt exerts time-dependent antibacterial activity, its continuous infusion is potentially beneficial. The efficacy of cefazolin for the treatment of bone and joint infections has been evaluated only once, by Fass. Cefazolin sodium salt was administered intermittently either intravenously or intramuscularly to 16 patients. Fifteen patients were considered cured, but only 6 of them were followed for at least 12 months. In our study, outcome analysis for 88 patients with at least 12 months of follow-up showed that 93% had no signs of infection. Five patients, all with S. aureus infections, relapsed with the same susceptible strain. No obvious cause of relapse (inappropriate surgery or antibiotic therapy, low serum cefazolin concentration, poor treatment compliance, severe immunodeficiency) could be identified. In conclusion, prolonged treatment of bone and joint infections with continuous intravenous cefazolin is feasible, effective, well-tolerated, safe, and convenient to administer, making continuous cefazolin sodium salt a strong candidate for home therapy.
Cefazolin sodium salt in the treatment of central nervous system infections
Infections of the central nervous system (CNS), including meningitis, encephalitis, ventriculitis, and brain abscesses, are particularly complex to treat and are generally associated with significant morbidity and mortality. CNS infections pose a significant treatment challenge due to the deep-seated condition, uncertainty regarding antibiotic exposure targets, and potential for adverse effects during antibiotic therapy. Among beta-lactams, antistaphylococcal penicillin antibiotics such as nafcillin and oxacillin are currently recommended in tertiary references and guidelines for treatment of CNS infections due to MSSA, while Cefazolin sodium salt is not preferred. However, the use of antistaphylococcal penicillins presents challenges with both administration and adverse events (e.g., fluid retention, elevated liver enzymes, interstitial nephritis) with protracted use. The use of Cefazolin sodium salt for CNS infections has become a topic of increased interest given superior tolerability, less frequent dosing, and mounting clinical data showing at least equivalent, if not improved, outcomes in the treatment of BSI and endocarditis when compared with antistaphylococcal penicillins. The objectives of this narrative review are to summarize the available data and produce a recommendation for Cefazolin sodium salt use in CNS infections.[2]
When comparing the PK properties of nafcillin, oxacillin, and cefazolin with literature reported values, all three agents exhibit constrained CSF penetration in the absence of meningeal inflammation. A rabbit model for pneumococcal meningitis was used to analyze Cefazolin sodium salt CSF concentrations at 2, 4, 6, and 8 h after a continuous 30 mg/kg/h dose of cefazolin. This analysis showed that cefazolin had a CSF penetration of 3.1%, which was similar to the penicillin G CSF penetration of 2.8%.Cefamandole and cephalothin had similar degrees of CSF penetration to penicillin G and cefazolin, likely a function of the high degree of serum protein binding (>50%) among these four agents. A case report of a patient with MSSA ventriculitis being successfully treated with high-dose Cefazolin sodium salt at 10 g/day followed by 8 g/day reported mean CSF concentrations of 11.9 and 6.1 mg/L, respectively, with no worsening in glomerular filtration rate. Both of these concentrations achieved 100% fT > MIC when considering the approved breakpoint for cefazolin (as surrogate to oxacillin/penicillin) against MSSA is ≤2 mcg/ml per the Clinical and Laboratory Standards Institute (CLSI). Another study among adult patients undergoing craniotomy reported that cefazolin achieved higher concentrations in homogenized brain tissue (mean: 10.6 mcg/g) than nafcillin or methicillin, at 30–225 min after a 2 g IV dose.
Pharmacological Study of Cefazolin During Intermittent and Continuous Infusion
Levels of cefazolin sodium salt were determined in plasma, urine, bile, and cerebrospinal fluid in humans after a bolus intravenous injection and during a controlled, continuous intravenous infusion. All the patients were studied in a steady-state and crossover fashion. With bolus injection and continuous infusion, respectively, 89.7 and 86.3% of the administered dose of cefazolin sodium salt were excreted in the urine of nine patients over the 6-h period considered. The levels of cefazolin in common bile duct bile were studied in six cholecystectomized patients. In bile collected during the two 3-h periods of the experiment, the mean concentration of the drug in the bile after bolus injection was 66.9 and 22.0 μg/ml, respectively; during continuous infusion, the corresponding biliary levels were 50.7 and 51.3 μg/ml, respectively.[3]
References
[1]Zeller V, Durand F, Kitzis MD, Lhotellier L, Ziza JM, Mamoudy P, Desplaces N. Continuous cefazolin infusion to treat bone and joint infections: clinical efficacy, feasibility, safety, and serum and bone concentrations. Antimicrob Agents Chemother. 2009 Mar;53(3):883-7. doi: 10.1128/AAC.00389-08. Epub 2008 Dec 15. PMID: 19075069; PMCID: PMC2650516.
[2]Antosz, Kayla et al. “Cefazolin in the treatment of central nervous system infections: A narrative review and recommendation.” Pharmacotherapy vol. 43,1 (2023): 85-95. doi:10.1002/phar.2750
[3]Thys JP, Vanderkelen B, Klastersky J. Pharmacological study of cefazolin during intermittent and continuous infusion: a crossover investigation in humans. Antimicrob Agents Chemother. 1976 Sep;10(3):395-8. doi: 10.1128/AAC.10.3.395. PMID: 984782; PMCID: PMC429759.
Lastest Price from Cefazolin sodium salt manufacturers

US $0.00/KG/Tin2025-04-21
- CAS:
- 27164-46-1
- Min. Order:
- 10KG
- Purity:
- 95%-102%; EP
- Supply Ability:
- 1000 KGS

US $10.00/KG2025-04-21
- CAS:
- 27164-46-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt