2-Methylbutane: toxicology, pharmacokinetics and applications
General Description
2-Methylbutane is a low-molecular-weight alkane derived from the petroleum raw materials natural gas and crude oil. It is a flammable liquid and has physical properties very similar to those of n-pentane. 2-Methylbutane is a skin irritant that can cause drying, cracking, and irritation with repeated direct contact. Similarities between 2-Methylbutane and n-pentane suggest similar behavior. It can remove natural skin oils and cause a chilling effect or freeze burn due to rapid evaporation. Ingesting 2-Methylbutane may result in nausea, vomiting, stomach pain, and aspiration hazards if swallowed, leading to respiratory complications. Inhalation studies on rats indicate anesthesia, tremors, and lung-related effects at high concentrations. 2-Methylbutane has diverse applications as a solvent, refrigerant, propellant, and fuel source but should be handled with caution due to flammability.

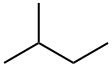
Figure 1. 2-Methylbutane
Toxicology
Skin Irritation
2-Methylbutane can be a skin irritant with potential adverse effects. Direct and repeated contact with the skin can lead to drying, cracking, and irritation. In studies involving human volunteers exposed to high quantities of n-pentane, which shares similarities with 2-Methylbutane, prolonged contact resulted in burning and blistering of the skin. It is important to note that solvents like 2-Methylbutane tend to remove the natural oils from the skin, and the rapid evaporation of pressurized liquid can cause a chilling effect or freeze burn. Therefore, proper caution and protective measures should be taken when handling or coming into contact with 2-Methylbutane. 1
Ingestion
Ingesting 2-Methylbutane is expected to result in symptoms such as nausea, vomiting, abdominal pain, and diarrhea. Due to its low surface tension and viscosity, 2-Methylbutane is also considered a potential aspiration hazard when swallowed. Once it enters the respiratory system and lungs, it can cause bronchial spasms, edema, hemorrhaging, chemical pneumonitis, asphyxia, or even death. Common signs and symptoms include coughing, choking, and gasping for breath. It is recommended not to induce vomiting if 2-Methylbutane is ingested, especially considering its low oral toxicity and the risk of aspiration. Prompt medical attention should be sought in such cases to ensure proper treatment and management of the individuals condition. 2
Inhalation
An inhalation study on rats exposed to 2-Methylbutane vapor was conducted. Four dose levels were tested, with concentrations ranging from 842 to 1842 mg/L air (equivalent to 285,453 to 624,471 ppm 2-Methylbutane). The calculated acute inhalation LC50 of 2-Methylbutane was 1281.9 mg/L air or 434,587 ppm 2-Methylbutane in air. Anesthesia occurred during exposure and persisted until death. Animals recovering survived high concentrations. Tremors were observed before death. In the 1423-mg/L group, lungs did not collapse upon necropsy, indicating a product-related effect. No other significant findings were noted. 3
Pharmacokinetics
The pulmonary absorption of 2-Methylbutane in animals was investigated. Male F344 rats were exposed to 1000-5000 ppm vapors of 2-Methylbutane for 80 to 100 minutes, and the uptake was found to be 8.6%. It was observed that highly volatile and branched hydrocarbons had lower absorption compared to less volatile and unbranched hydrocarbons. Unsaturated compounds showed better absorption than saturated ones. The metabolism of 2-Methylbutane was studied, and it was found that rat, mouse, rabbit, and guinea pig liver microsomes hydroxylate 2-Methylbutane, producing 2-methyl-2-butanol as the major metabolite, along with minor metabolites such as 3-methyl-2-butanol, 2-methyl-1-butanol, and 3-methyl-1-butanol. However, the in vivo metabolism of 2-Methylbutane has not been reported. 2
Applications
2-Methylbutane has diverse applications across industries. It is utilized as a solvent for extraction and purification in laboratories, as well as a refrigerant in cooling systems. 2-Methylbutane serves as an aerosol propellant for products like spray paints and insecticides. Additionally, it acts as a building block in chemical synthesis for plastics, synthetic rubber, and pharmaceutical intermediates. 2-Methylbutane can also function as a fuel source for camping stoves and portable heaters. However, precautionary measures must be taken due to its flammable properties.
Reference
1. American Conference of Governmental Industrial Hygienists. Documentation of the threshold limit values and biological exposure indices, 6th ed. Cincinnati, OH: ACGIH, 1991.
2. Galvin JB, Marashi F. 2-Methylbutane (isopentane). CAS# 78-78-4. J Toxicol Environ Health A, 1999, 58(1-2):23-33.
3. Phillips Petroleum Co. Isopentane Toxicity Study Summary. Bartlesville, OK. Hazelton Laboratories America, Inc, 1982.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Methylbutane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 78-78-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $23.00/L2025-01-16
- CAS:
- 78-78-4
- Min. Order:
- 1L
- Purity:
- 0.99
- Supply Ability:
- 3000000tons