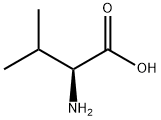
L-Valine: Applications, metabolism and medical significance
General description
L-Valine (symbol Val or V) is an α-amino acid, used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans, and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). Like leucine and isoleucine, L-valine is a branched-chain amino acid. In sickle-cell disease, a single glutamic acid in β-globin is replaced by L-valine. Because L-valine is hydrophobic, whereas glutamic acid is hydrophilic, this change makes the hemoglobin prone to abnormal aggregation. L-Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name L-valine comes from valeric acid, which in turn is named after the plant valerian. Its appearance is as follows:

Figure 1 Appearance of L-Valine
Applications
L-Valine works with two other high-concentration amino acids (Isoleucine and Leucine) to promote growth of the body, repair tissues, regulate blood sugar, and provide needed energy. In addition, the role of L-Valine also includes stimulating the central nervous system, which is necessary for proper mental operation. When participating in intense physical activities, L-Valine can provide extra energy for muscles to produce glucose, so as to prevent muscle weakness. It also helps remove excess nitrogen (potential toxins) from the liver and transport the nitrogen needed by the body to various parts. L-Valine may also be useful in the treatment of liver and gallbladder diseases, as well as the damage to these organs caused by alcohol and drug abuse.
Metabolism
L-Valine, like other branched-chain amino acids, is synthesized by plants, but not by animals.[1] It is an essential amino acid in animals, and needs to be present in the diet. Adult humans require about 4 mg/kg body weight daily. It is synthesized in plants and bacteria via several steps starting from pyruvic acid. The initial part of the pathway also leads to leucine. The intermediate α-ketoisovalerate undergoes reductive amination with glutamate. Enzymes involved in this biosynthesis include Acetolactate synthase, Acetohydroxy acid reductase, Dihydroxyacid dehydratase, and Valine aminotransferase. Like other branched-chain amino acids, the catabolism of L-valine starts with the removal of the amino group by transamination, giving alpha-ketoisovalerate, an alpha-keto acid, which is converted to iso butyryl-CoA through oxidative decarboxylation by the branched-chain α-ketoacid dehydrogenase complex.[2] This is further oxidized and rearranged to succinyl-CoA, which can enter the citric acid cycle.
Medical significance
L-Valine, like other branched-chain amino acids, is associated with insulin resistance: higher levels of valine are observed in the blood of diabetic mice, rats, and humans.[3] Mice fed a valine deprivation diet for one day have improved insulin sensitivity, and feeding of a valine deprivation diet for one week significantly decreases blood glucose levels. In diet-induced obese and insulin-resistant mice, a diet with decreased levels of L-valine and the other branched-chain amino acids results in reduced adiposity and improved insulin sensitivity.[4] The valine catabolite 3-hydroxybutyrate promotes skeletal muscle insulin resistance in mice by stimulating fatty acid uptake into muscle and lipid accumulation. In humans, a protein-restricted diet lowers blood levels of L-valine and decreases fasting blood glucose levels. Dietary L-valine is essential for hematopoietic stem cell (HSC) self-renewal, as demonstrated by experiments in mice.[5] Dietary L-valine restriction selectively depletes long-term repopulating HSC in mouse bone marrow. Successful stem cell transplantation was achieved in mice without irradiation after 3 weeks on a L-valine restricted diet. Long-term survival of the transplanted mice was achieved when L-valine was returned to the diet gradually over a 2-week period to avoid refeeding syndrome.
References
[1]Basuchaudhuri, Pranab (2016). Nitrogen metabolism in rice. Boca Raton, Florida: CRC Press. p. 159. ISBN 9781498746687. OCLC 945482059.
[2]Mathews, Christopher K. (2000). Biochemistry. Van Holde, K. E., Ahern, Kevin G. (3rd ed.). San Francisco, Calif.: Benjamin Cummings. p. 776. ISBN 0805330666. OCLC 42290721.
[3]Lynch, Christopher J.; Adams, Sean H. (1 December 2014). "Branched-chain amino acids in metabolic signalling and insulin resistance". Nature Reviews. Endocrinology. 10 (12): 723–736. doi:10.1038/nrendo.2014.171. ISSN 1759-5037. PMC 4424797. PMID 25287287.
[4]Cummings, Nicole E.; Williams, Elizabeth M.; Kasza, Ildiko; Konon, Elizabeth N.; Schaid, Michael D.; Schmidt, Brian A.; Poudel, Chetan; Sherman, Dawn S.; Yu, Deyang (19 December 2017). "Restoration of metabolic health by decreased consumption of branched-chain amino acids". The Journal of Physiology. 596 (4): 623–645. doi:10.1113/JP275075. ISSN 1469-7793. PMC 5813603. PMID 29266268.
[5]Taya, Yuki; Ota, Yasunori; Wilkinson, Adam C.; Kanazawa, Ayano; Watarai, Hiroshi; Kasai, Masataka; Nakauchi, Hiromitsu; Yamazaki, Satoshi (2 December 2016). "Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation". Science. 354 (6316): 1152–1155. Bibcode:2016Sci...354.1152T. doi:10.1126/science.aag3145. PMID 27934766. S2CID 45815137.
You may like
Related articles And Qustion
See also
Lastest Price from L-Valine manufacturers

US $0.00-0.00/kgs2025-06-19
- CAS:
- 72-18-4
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons

US $0.00-0.00/kgs2025-06-19
- CAS:
- 72-18-4
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons