Anastrozole: Applications, pharmacology and side effects
General description
Anastrozole, sold under the brand name Arimidex among others, is a medication used in addition to other treatments for breast cancer. Specifically, it is used for hormone receptor-positive breast cancer. It has also been used to prevent breast cancer in those at high risk. It is taken by mouth. Common side effects of anastrozole include hot flashes, altered mood, joint pain, and nausea. Severe side effects include an increased risk of heart disease and osteoporosis. Use during pregnancy may harm the baby. Anastrozole is in the aromatase-inhibiting family of medications. It works by blocking the production of estrogens in the body and hence has antiestrogenic effects. Anastrozole was patented in 1987 and was approved for medical use in 1995. It is on the World Health Organization's List of Essential Medicines. Anastrozole is available as a generic medication. In 2017, it was the 258th most commonly prescribed medication in the United States, with more than one million prescriptions. Its appearance is as follows:

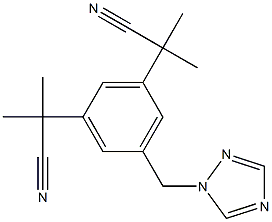
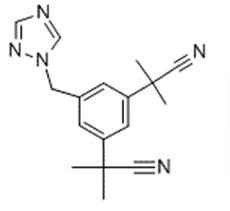
Figure 1 Appearance of Anastrozole
Applications
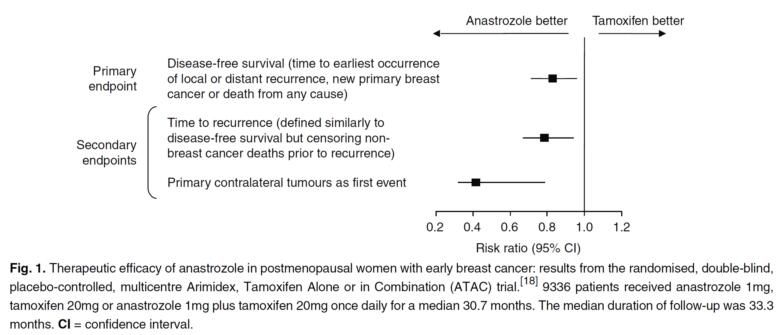
Anastrozole is used in the treatment and prevention of breast cancer in women.[1] The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial was of localized breast cancer and women received either anastrozole, the selective estrogen receptor modulator tamoxifen, or both for five years, followed by five years of follow-up.[2] After more than 5 years the group that received anastrozole had better results than the tamoxifen group.[2] The trial suggested that anastrozole is the preferred medical therapy for postmenopausal women with localized estrogen receptor-positive breast cancer.[2] Anastrozole is used at a dosage of 0.5 to 1 mg/day in combination with the antiandrogen bicalutamide in the treatment of peripheral precocious puberty, for instance, due to familial male-limited precocious puberty (testotoxicosis) and McCune–Albright syndrome, in boys.
Pharmacology
Anastrozole works by reversibly binding to the aromatase enzyme, and through competitive inhibition block the conversion of androgens to estrogens in peripheral (extragonadal) tissues. The medication has been found to achieve 96.7% to 97.3% inhibition of aromatase at a dosage of 1 mg/day and 98.1% inhibition of aromatase at a dosage of 10 mg/day in humans.[3] As such, 1 mg/day is considered to be the minimal dosage required to achieve maximal suppression of aromatase with anastrozole.[3] This decrease in aromatase activity results in an at least 85% decrease in estradiol levels in postmenopausal women.[3] Levels of corticosteroids and other adrenal steroids are unaffected by anastrozole.[3]
The bioavailability of anastrozole in humans is unknown, but it was found to be well-absorbed in animals. Absorption of anastrozole is linear over a dosage range of 1 to 20 mg/day in humans and does not change with repeated administration.[3][4] Food does not significantly influence the extent of absorption of anastrozole.[4] Peak levels of anastrozole occur a median of 3 hours after administration, but with a wide range of 2 to 12 hours.[4] Steady-state levels of anastrozole are achieved within 7 to 10 days of continuous administration, with 3.5-fold accumulation.[3][4] However, maximal suppression of estradiol levels occurs within 3 or 4 days of therapy.[3] Active efflux of anastrozole by P-glycoprotein at the blood–brain barrier has been found to limit the central nervous system penetration of anastrozole in rodents, whereas this was not the case with letrozole and vorozole. As such, anastrozole may have peripheral selectivity in humans, although this has yet to be confirmed. In any case, estradiol is synthesized peripherally and readily crosses the blood–brain barrier, so anastrozole would still expect to reduce estradiol levels in the central nervous system to a certain degree. The plasma protein binding of anastrozole is 40%.[3][4]
Side effects
Common side effects of anastrozole (≥10% incidence) include hot flashes, asthenia, arthritis, pain, arthralgia, hypertension, depression, nausea and vomiting, rash, osteoporosis, bone fractures, back pain, insomnia, headache, bone pain, peripheral edema, coughing, dyspnea, pharyngitis, and lymphedema. Serious but rare adverse effects (<0.1% incidence) include skin reactions such as lesions, ulcers, or blisters; allergic reactions with swelling of the face, lips, tongue, and/or throat that may cause difficulty swallowing or breathing; and abnormal liver function tests as well as hepatitis.
References
[1]"Anastrozole". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
[2]Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. (2005). "Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer". Lancet. 365 (9453): 60–2. doi:10.1016/S0140-6736(04)17666-6. PMID 15639680.
[3]Lønning P, Pfister C, Martoni A, Zamagni C (August 2003). "Pharmacokinetics of third-generation aromatase inhibitors". Seminars in Oncology. 30 (4 Suppl 14): 23–32. doi:10.1016/S0093-7754(03)00305-1. PMID 14513434.
[4]Sanford M, Plosker GL (2008). "Anastrozole: a review of its use in postmenopausal women with early-stage breast cancer". Drugs. 68 (9): 1319–40. doi:10.2165/00003495-200868090-00007. PMID 18547136.
You may like
Related articles And Qustion
See also
Lastest Price from Anastrozole manufacturers

US $0.00/Box2025-09-26
- CAS:
- 120511-73-1
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 10 tons

US $10.00/box2025-08-22
- CAS:
- 120511-73-1
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock