Anastrozole: Pharmacological Properties, Therapeutic Efficacy and Tolerability
General Description
Anastrozole is a competitive inhibitor of aromatase, reducing estrogen production in postmenopausal women and breast cancer patients. Clinical trials show that anastrozole is more efficacious than tamoxifen in achieving disease-free survival and reducing recurrence rates. Anastrozole has a favorable tolerability profile compared to tamoxifen, with fewer treatment-related adverse events. However, anastrozole may cause decreased bone mineral density and increased levels of serum cholesterol. Overall, anastrozole is an effective and well-tolerated treatment option for early-stage breast cancer in postmenopausal women, but healthcare providers should monitor patients for potential adverse effects.
Figure 1. Tablets of anastrozole
Pharmacological Properties
Anastrozole is a benzyltriazole derivative that acts as a competitive inhibitor of the enzyme aromatase, which is responsible for estrogen synthesis. By binding to the haem group of the cytochrome P450 unit of aromatase, anastrozole reduces the production of estrogen. It is a potent and selective inhibitor of aromatase, with minimal effects on adrenal steroid hormones. In postmenopausal women, anastrozole has been shown to effectively suppress plasma estradiol levels by at least 80% from baseline. In patients with advanced breast cancer, both plasma and tumor tissue levels of estradiol can be reduced by approximately 90% compared to baseline. Clinical trials have demonstrated that long-term use of anastrozole may lead to decreased bone mineral density in the hip and lumbar spine, as well as increased levels of serum cholesterol. However, unlike tamoxifen, anastrozole does not cause thickening of the endometrium after 5 years of treatment. Anastrozole exhibits linear pharmacokinetics and is primarily metabolized in the liver. Its plasma elimination half-life is approximately 40-50 hours, indicating that once-daily administration is sufficient. Studies suggest that anastrozole has minimal potential for drug-drug interactions with medications metabolized by hepatic cytochrome P450 enzymes, including tamoxifen. In conclusion, anastrozole is an effective and well-tolerated aromatase inhibitor that significantly reduces estrogen levels in postmenopausal women and breast cancer patients. 1
Therapeutic Efficacy
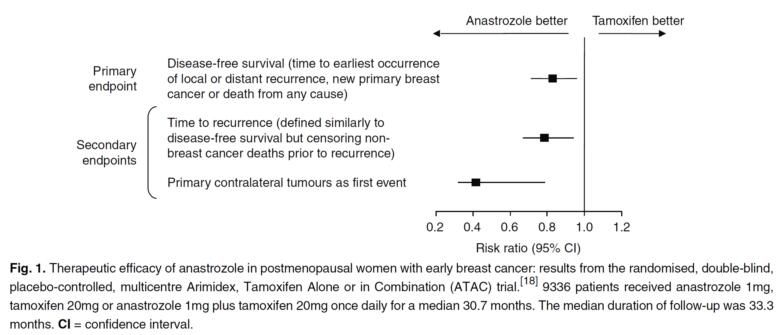
The therapeutic efficacy of anastrozole has been extensively studied in clinical trials, particularly in the treatment of early-stage breast cancer in postmenopausal women. The ATAC trial, a randomized and double-blind study, compared the effectiveness of anastrozole versus tamoxifen as primary adjuvant treatments. Results consistently showed that anastrozole was more efficacious than tamoxifen in achieving both primary and secondary endpoints at various follow-up periods of 3 years, 5 years, and up to 100 months. At the 100-month follow-up, anastrozole demonstrated superior disease-free survival compared to tamoxifen, with a hazard ratio of 0.85 in the hormone receptor-positive population. This represents a 4.1% absolute difference in the event rate, indicating the significant benefit of anastrozole over tamoxifen. Other trials have explored the efficacy of switching from tamoxifen to anastrozole after 2-3 years of tamoxifen treatment. These studies found that patients who switched to anastrozole had lower odds of experiencing breast cancer recurrence, contralateral breast cancer, or death compared to those who continued with tamoxifen. In another trial, extended treatment with anastrozole following 5 years of tamoxifen therapy resulted in fewer recurrences, particularly distant recurrences, compared to patients who did not receive extended treatment. Overall, these trials demonstrate the therapeutic superiority of anastrozole in terms of disease-free survival and reduction in recurrence rates, supporting its use as an effective treatment option for postmenopausal women with early-stage breast cancer. 2
Tolerability
Anastrozole has been shown to be generally well tolerated as a primary adjuvant treatment for early-stage breast cancer, according to the ATAC trial. In fact, patients who received anastrozole had significantly fewer treatment-related adverse events, serious adverse events, and withdrawals due to adverse events compared to those who received tamoxifen. However, some adverse events were more common in the anastrozole group, including osteopenia/osteoporosis, arthralgia, fractures, hypertension, hypercholesterolemia, diarrhea, and paraesthesiae. On the other hand, gynecological events such as hot flushes, vaginal discharge, vaginal bleeding, endometrial cancer, and hysterectomy were significantly more common in the tamoxifen group, as were urinary tract infections, muscle cramps, anemia, venous thrombosis, deep vein thrombosis, and ischaemic cerebrovascular events. These findings were consistent with the results of switching trials that evaluated the tolerability of switching from tamoxifen to anastrozole after 2-3 years of tamoxifen treatment. Overall, anastrozole was well-tolerated as a primary adjuvant treatment for early-stage breast cancer, and its tolerability profile was generally favorable compared to tamoxifen. However, healthcare providers should carefully monitor patients for potential adverse effects and discuss the benefits and risks of treatment options with their patients. 1
Reference
1. Sanford M, Plosker GL. Anastrozole: a review of its use in postmenopausal women with early-stage breast cancer. Drugs. 2008;68(9):1319-1340.
2. Kelly CM, Buzdar AU. Anastrozole. Expert Opin Drug Saf. 2010;9(6):995-1003.
Related articles And Qustion
Lastest Price from Anastrozole manufacturers

US $0.00/Box2025-09-26
- CAS:
- 120511-73-1
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 10 tons

US $10.00/box2025-08-22
- CAS:
- 120511-73-1
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock