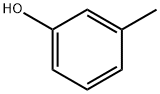
m-Cresol- Reaction / Application on Synthetic Works
m-Cresol is an important organic intermediate (building block) to synthetize substituted aryl products.
The following example is about its application on the synthesis of coumarin derivatives [1].
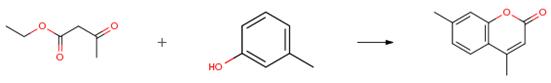
A mixture of resorsinol (1 mmol), ethyl acetoacetate (1 mmol), and FeF3 (0.05 g) was ground in an open Pyrex beaker and the homogenized mixture was heated by microwave irradiation for about 7 min. The progress of the reaction was monitored by using TLC (ethylacetate/n-hexane: 1/2). After complete conversion as indicated by TLC, the mixture was extracted with petroleum ether (3 × 30 mL) and washed with water (3 × 30 mL). The crude products were purified by recrystallization from ethanol (95%) to afford pure products.
The following example is about its application on the synthesis of aryl tosylates and mesylates [2].
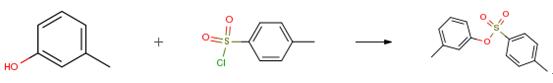
To a solution of hydroxyarene (25.0 mmol) in THF (15 mL) was added 10 percent K2CO3 (65.00 g, 47.1 mmol) or 15 percent NaOH (22.00 g, 82.5 mmol). After the resulting solution was cooled to 0 °C with an ice-water bath, a solution of TsCl (4.82 g, 25.3 mmol for 10percent K2CO3 or 5.72 g, 30.0 mmol for 15 percent NaOH) in THF (35 mL) was slowly added within 15 min at 0 °C. After addition of TsCl, the ice-water bath was removed and the reaction mixture was stirred for 2 h. To the mixture was then added ethyl acetate (100 mL; more was needed when aryl tosylate has poor solubility in ethyl acetate). The two-phase mixture was separated. The organic layer was washed with water (50 mL) and dried over anhydrous MgSO4. Removal of solvent under reduced pressure gave the corresponding pure aryl tosylate. Any trace amount of TsCl, if present, can be removed by simply washing the product with hexanes.
The following example is about its application on the synthesis of mucohalic acids [3].
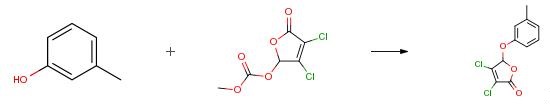
The masked mucohalic acid (2.0 mmol) was combined with m-cresol (Formula 10, 1.1 equiv.), base (0.15-0.50 equiv.), Pd catalyst (0 or 3 mol percent), and dichloromethane (20 mL) and was stirred at RT for 4 h to 65 h, depending on the reaction conditions. The reaction was quenched with saturated aqueous ammonium chloride (20 mL) and partitioned between water (20 μL) and dichloromethane (80 mL). The organic extract was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/heptane) to give 2,3-dichloro-4-m-tolyloxy-2-buten-4-olide
The following example is about its application on vinylation of phenols and naphthols with acetylene [4].

In the reactor (0.3 L) was charged 50 mmol of ArOH 1, 1.62 g (25 mmol) of KOH•0.5H2O, 50 mL of DMSO, the reactor was closed gas-tight, the air was replaced by nitrogen, and acetylene was charged into the reactor to initial pressure 13–15 at. The reaction mixture at vigorous stirring (240 rpm) was heated at 120°C. The pressure in the reactor at the moment when the temperature reached the desired value was 15–16 at and decreased in the course of the reaction. At the completion of the experiment the reactor was cooled, the reaction mixture was diluted with 1 percent water solution of NH4Cl (100 mL), the reaction products were extracted with Et2O (6 × 30 mL). The extract was dried with Na2SO4, the solvent was distilled off on a rotary evaporator. The residue was distilled in a vacuum to obtain arylvinyl ethers. Divinyl ethers was obtained similarly from dihydric phenols (50 mmol) and acetylene.
References
1.Vahabi V, Hatamjafari F. Microwave assisted convenient one-pot synthesis of coumarin derivatives via Pechmann condensation catalyzed by FeF3 under solvent-free conditions and antimicrobial activities of the products[J]. Molecules, 2014,19(9):13093-13103.
2.Lei X, Jalla A, Abou S, Mhd A, Stafford JM, Cao B. Chromatography-Free and Eco-Friendly Synthesis of Aryl Tosylates and Mesylates[J]. Synthesis, 2015, 47(17): 2578 - 2585
3.Blazecka PG, Zhang J. Preparation of substituted butenolides via palladium-free etherification and amination of masked mucohalic acids. US2005/59831[P], 2005, A1, Location in patent: Page/Page column 7-8
4.Trofimov OK, Vysotskaya G. Nucleophilic addition to acetylenes in superbasic catalytic systems: XVIII. Vinylation of phenols and naphthols with acetylene[J]. Russian Journal of Organic Chemistry, 2015, 51(2):188 – 194
You may like
Related articles And Qustion
Lastest Price from m-Cresol manufacturers

US $0.00/kg2025-03-07
- CAS:
- 108-39-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $10.00/KG2024-10-11
- CAS:
- 108-39-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton