Application research of 2-Bromophenylboronic acid
Introduction
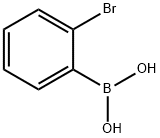
2-Bromophenylboronic acid (Figure.1) serves as a crucial intermediate for electronic chemicals used in Organic Light-Emitting Diodes (OLEDs). As an emerging display technology, OLED have witnessed rapid development in recent years, achieving widespread commercial applications. It is anticipated that in the foreseeable future, OLEDs will supplant existing display technologies and permeate every aspect of production and daily life. Organic phenylboronic acid compounds, as vital intermediates for their luminescent materials, have garnered significant attention from chemists, leading to extensive research and development efforts in this field.[1]

2-Bromophenylboronic acid Preparation
First o-bromo-iodobenzene was synthesized from o-bromoaniline through diazotization and iodination, the yield was 83%(HPLC purity 99.5%), and then o-bromophenyl magnesium bromide was synthesized by the reaction of o-bromo-iodobenzene and isopropyl magnesium bromide. Finally 2-bromophenylboronic acid was synthesized through the nucleophilic substitution of o-bromophenyl magnesium bromide with trimethyl borate and then the hydrolysis, the yield of 59.9% ( HPLC purity of 99.6% ) was obtained.[1]
Application research example
Indenones Preparation
The Rh-catalyzed reaction of alkynes with 2-bromophenylboronic acids involves carbonylative cyclization to give indenones. The key steps in the reaction involve the addition of an arylrhodium(I) species to an alkyne and the oxidative addition of C-Br bonds on the adjacent phenyl ring to give vinylrhodium(I) species II. The regioselectivity depends on both the electronic and the steric nature of the substituents on the alkynes. A bulky group and an electron-withdrawing group favor the -position of indenones. In the case of silyl- or ester-substituted alkynes, the regioselectivity is extremely high. The selectivity increases in the order SiMe3 > COOR >> aryl >> alkyl. The reaction of norbornene with 2-bromophenylboronic acids under 1 atm of CO gives the corresponding indanone derivative. The reaction of alkynes with 2-bromophenylboronic acids under nitrogen gives naphthalene derivatives, in which two molecules of alkynes are incorporated. A vinylrhodium complex similar to II can also be generated by a different route by employing 2-bromophenyl(trimethylsilyl)acetylene and arylboronic acids in the presence of Rh(I) complex as the catalyst, resulting in the formation of indenones. The reaction of 1-(2-bromophenyl)-hept-2-yn-1-one with PhB(OH)2 in the presence of Rh(I) complex also resulted in carbonylative cyclization to give an indan-1,3-dione derivative.[2]
Novel core-expanded rylenebis(dicarboximide) dyes Preparation
Two synthetic routes for the benzannulation in the "bay"-region of rylenebis(dicarboximide)s leading to new pi-system-expanded chromophores are described. The first route follows a two-step approach: Suzuki coupling of bromo-substituted perylenebis(dicarboximide) with 2-bromophenylboronic acid, followed by palladium-catalysed dehydrobromination. The second route is best described as a palladium-assisted cycloaddition of benzyne, formed in situ, to the bay-region of the bromo-substituted rylene core. Two new types of core-expanded rylene dyes were synthesised: yellow dibenzocoronenebis(dicarboximide)s, absorbing at 490 nm, and a green dinaphthoquaterrylenebis(dicarboximide), which absorbs at 700 nm. These new chromophores are characterised by significant hypsochromic shifts of absorption, compared to their parent rylenebis(dicarboximide)s, excellent photostabilities and high fluorescence quantum yields.[3]
Other studies in organic synthesis
1. A new approach for the convenient synthesis of dibenzazepinones is reported. The key step is the formation of the seven-membered ring through palladium-catalyzed intramolecular arylation of an anilide enolate. The reactions were completed in 10 min at 100 °C with moderate to excellent yields. Aminodibenzazepinone 1, the core structure in the γ-secretase inhibitor LY411575, can be prepared in five steps from 2-bromophenylboronic acid and 2-iodoaniline in 60% overall yield. The synthesis reported here compares favorably with presently available approaches to this interesting ring system.[4]
2. Intramolecular C-H arylations were employed as a key step in the synthesis of hitherto unknown fused purine systems: 13-substituted purino[8,9-f]phenanthridines and 11-substituted 5,6-dihydropurino[8,9-a]isoquinolines. The purino[8,9-f]phenanthridines were prepared in moderate yields by double C-H arylations of 9-phenylpurines with 1,2-diiodobenzene or, more efficiently, by consecutive Suzuki coupling of 9-(2-bromophenyl)purines with 2-bromophenylboronic acid followed by intramolecular C-H arylation. 5,6-Dihydropurino[8,9-a]isoquinolines were prepared in quantitative yields by intramolecular C-H arylations of 9-(2-chlorophenethyl)purines.[5]
3. The rhodium(I)-catalyzed reaction of alkynes with 2-bromophenylboronic acids in the presence of paraformaldehyde resulted in a CO gas-free carbonylative cyclization, yielding indenone derivatives. [RhCl(BINAP)](2) and [RhCl(cod)](2) were responsible for the decarbonylation of formaldehyde and the subsequent carbonylation of alkynes with 2-haloboronic acids, respectively, leading to efficient whole carbonylation. Sterically bulky and electron-withdrawing groups on unsymmetrically substituted alkynes favored the alpha-position of indenones.[6]
4. Palladium serves as a multi-functional catalyst which is controllable by tuning reaction conditions. This work demonstrated the utilization of a palladium catalyst for the synthesis of phenanthrenols by cascade palladium-catalyzed Suzuki/Heck reaction between chalcone and 2-bromophenylboronic acid, followed by Michael addition. The sequential reaction could be controlled by reactivity of the palladium catalyst in different solvents and concentrations of reagents. This protocol could be applied to a broad range of substrates to give products in low to good yields.[7]
References
1. Liu Bing-wen,et al.Study on Preparation of o-Bromophenyl Boronic Acid[J].辽宁化工,2014,43(11):1385-1386+1395.
2. Harada Y, Nakanishi J, Fujihara H, Tobisu M, Fukumoto Y, Chatani N. Rh(I)-catalyzed carbonylative cyclization reactions of alkynes with 2-bromophenylboronic acids leading to indenones. J Am Chem Soc. 2007;129(17):5766-5771. doi:10.1021/ja070107n
3. Avlasevich Y, Müller S, Erk P, Müllen K. Novel core-expanded rylenebis(dicarboximide) dyes bearing pentacene units: facile synthesis and photophysical properties. Chemistry. 2007;13(23):6555-6561. doi:10.1002/chem.200700523
4. Pan X, Wilcox CS. Synthesis of dibenzazepinones by palladium-catalyzed intramolecular arylation of o-(2'-bromophenyl)anilide enolates. J Org Chem. 2010;75(19):6445-6451. doi:10.1021/jo101137r
5. Cerna I, Pohl R, Klepetárová B, Hocek M. Intramolecular direct C-H arylation approach to fused purines. Synthesis of purino[8,9-f]phenanthridines and 5,6-dihydropurino[8,9-a]isoquinolines. J Org Chem. 2010;75(7):2302-2308. doi:10.1021/jo100111t
6. Morimoto T, Yamasaki K, Hirano A, et al. Rh(I)-catalyzed CO gas-free carbonylative cyclization reactions of alkynes with 2-bromophenylboronic acids using formaldehyde. Org Lett. 2009;11(8):1777-1780. doi:10.1021/ol900327x
7.Yongpanich P, Chatrangsan K, Tummatorn J, Thongsornkleeb C, Ruchirawat S. Controllable Chemoselectivity Cascade Reactions for the Synthesis of Phenanthrenols via Palladium-Catalyzed-Suzuki/Heck Reaction and Michael Addition. Chem Asian J. 2024;19(9):e202400126. doi:10.1002/asia.202400126
You may like
See also
Lastest Price from 2-Bromophenylboronic acid manufacturers
US $34.00-1.20/kg2024-04-08
- CAS:
- 244205-40-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $48.00-40.00/KG2023-05-04
- CAS:
- 244205-40-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200tons