Application research of Pyridine-3-Sulfonyl Chloride
Introduction
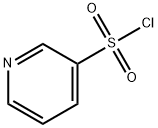
Pyridine-3-sulfonyl chloride (Figure 1) exists primarily as a colorless to pale yellow liquid at room temperature, and can also be found in the form of an off-white to pale yellow crystalline powder. Its applications are mainly reflected in the following three aspects: (1) Pyridine-3-sulfonyl chloride serves as a key intermediate for synthesizing a variety of pharmaceuticals, such as antibacterial agents, anti-inflammatory drugs, and therapeutics for cardiovascular diseases. Its derived sulfonamide compounds usually exhibit excellent biological activity, and can act as enzyme inhibitors or receptor antagonists. (2) Pyridine-3-sulfonyl chloride is used in the synthesis of highly effective and low-toxicity pesticides, including herbicides and insecticides. By modifying the structures of the pyridine ring and sulfonyl group, the targeting ability and degradation performance of pesticides can be optimized. (3) As a sulfonylation reagent, pyridine-3-sulfonyl chloride can be employed to introduce the pyridine-3-sulfonyl group into organic molecules, thereby regulating the solubility, acidity and biological activity of the molecules. It is widely applied in the synthesis and modification of heterocyclic compounds. This paper provides a comprehensive review of the application research of pyridine-3-sulfonyl chloride.

Bisphenol A as degradation product of monomers
There is still much debate about the release of bisphenol-A (BPA) from dental materials. Therefore, this study aimed to quantify BPA present as an impurity in both BPA-based and non-BPA-based monomers and to evaluate whether these monomers may degrade to BPA upon salivary, bacterial, and chemical challenges. BPA was determined in three different amounts (1, 2, and 3 μmol) of each monomer (TEGDMA, UDMA, mUDMA, BisGMA, BisEMA-3, -6, -10, -30, BisPMA, EBPADMA urethane, BADGE, and BisDMA). Next, the monomers (3 μmol) were immersed in whole human pooled saliva collected from adults, Streptococcus mutans (2 × 107 CFU/mL), and acidic (0.1 M HCl), alkaline (0.1 M NaOH), and control media. The amount of BPA was quantified using a specific and highly sensitive UPLC-MS/MS method including derivatization of BPA by pyridine-3-sulfonyl chloride. The monomers BisGMA and BisEMA-3 contained trace amounts (0.0006% and 0.0025%, respectively) of BPA as impurities of their synthesis process. BPA concentrations increased when the monomers BisGMA, BisEMA-3, BisEMA-6, BisEMA-10, BisPMA and BADGE were exposed to saliva and S. mutans, indicating degradation of a small amount of monomer into BPA. In addition, BisPMA and BADGE degraded into BPA under alkaline conditions. The conversion rate of the monomers into BPA ranged between 0.0003% and 0.0025%. Impurities and degradation of BPA-based monomers may account for the release of BPA from resin-based dental materials. Even though the detected amounts of BPA due to monomer impurity were small, manufacturers of dental materials can reduce the BPA content by using only monomers of the highest purity. Considering the overall current trend towards BPA-free materials, it may be recommendable to investigate whether non-BPA based monomers can be used in dental resin-based materials.[1]
Level of BPA contamination in resin composites determines BPA release
Resin composites may release bisphenol A (BPA) due to impurities present in the monomers. However, there is a lack of knowledge regarding the leaching characteristics of BPA from resin composites. Therefore, experimental resin composites were prepared with known amounts of BPA. The objective of this study was (1) to determine which amount of BPA initially present in the material leaches out in the short term and, (2) how this release is influenced by the resin composition. BPA (0, 0.001, 0.01, or 0.1 wt%) was added to experimental resin composites containing 60 mol% BisGMA, BisEMA(3), or UDMA, respectively, as base monomer and 40 mol% TEGDMA as diluent monomer. Polymerized samples (n = 5) were immersed at 37 °C for 7 days in 1 mL of water, which was collected and refreshed daily. BPA release was quantified with UPLC-MS/MS after derivatization with pyridine-3-sulfonyl chloride. Between 0.47 to 0.67 mol% of the originally added BPA eluted from the resin composites after 7 days. Similar elution trends were observed irrespective of the base monomer. Two-way ANOVA showed a significant effect of the base monomer on BPA release, but the differences were small and not consistent.
The released amount of BPA was directly proportional to the quantity of BPA present in the resin composite as an impurity. BPA release was mainly diffusion-based, while polymer composition seemed to play a minor role. Our results underscore the importance for manufacturers only to use monomers of the highest purity in dental resin composites to avoid unnecessary BPA exposure in patients.[2]
Highly Sensitive and High-Throughput Method exploration
Study 1:The structural analogs of bisphenol A (BPA) and their halogenated derivatives (together termed BPs) have been found in the environment, food, and even the human body. Limited research showed that some of them exhibited toxicities that were similar to or even greater than that of BPA. Therefore, adverse health effects for BPs were expected for humans with low-dose exposure in early life. Breast milk is an excellent matrix and could reflect fetuses' and babies' exposure to contaminants. Some of the emerging BPs may present with trace or ultratrace levels in humans. However, existing analytical methods for breast milk cannot quantify these BPs simultaneously with high sensitivity using a small sampling weight, which is important for human biomonitoring studies. In this paper, a method based on Bond Elut Enhanced Matrix Removal-Lipid purification, pyridine-3-sulfonyl chloride derivatization, and liquid chromatography electrospray tandem mass spectrometry was developed. The method requires only a small quantity of sample (200 μL) and allowed for the simultaneous determination of 24 BPs in breast milk with ultrahigh sensitivity. The limits of quantitation of the proposed method were 0.001-0.200 μg/L, which were 1-6.7 times lower than the only study for the simultaneous analysis of bisphenol analogs in breast milk based on a 3 g sample weight. The mean recoveries ranged from 86.11% to 119.05% with relative standard deviation (RSD)≤19.5% (n=6). Matrix effects were within 20% with RSD<10% for six different lots of samples. The proposed method was successfully applied to 20 breast milk samples. BPA, bisphenol F (BPF), bisphenol S (BPS), and bisphenol AF (BPAF) were detected. BPA was still the dominant BP, followed by BPF. This is the first report describing the occurrence of BPF and BPAF in breast milk.[3]
Study 2: A reliable and sensitive analyzing method was developed and validated for determination of 13 novel bisphenol analogues (BPs) along with bisphenol A (BPA) in organism tissues. The complex organism tissues were treated by ultrasonic-assisted extraction using acetonitrile/formic acid (99:1, v/v), followed by successive purification using enhanced matrix removal-lipid sorbents and primary secondary amine sorbents. The BPs were finally determined by ultra-high performance liquid chromatography-tandem mass spectrometry after derivatization using pyridine-3-sulfonyl chloride. Satisfactory recoveries of 75-118% were obtained for the BPs, with good repeatability (RSD<20%). Matrix interferences were efficiently diminished. The method quantification limits (MQLs) reached 0.003-0.1ng/g dry weight (dw). The validated method was successfully applied to a preliminary investigation of the BPs in wild marine organisms collected from the nearshore waters along the coast of Guangdong, China. Besides BPA, novel BPs such as bisphenol F, bisphenol AF, and tetrabromobisphenol A were also detected at < MDL-15.5 ng/g dw. This work laid a strong basis for further in-depth research on bioaccumulation of the novel BPs in the environment.[4]
References
[1] De Nys S, Duca RC, Vervliet P, et al. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent Mater. 2021;37(6):1020-1029. doi:10.1016/j.dental.2021.03.005
[2] De Nys S, Turkalj M, Duca RC, et al. Level of BPA contamination in resin composites determines BPA release. Dent Mater. 2024;40(7):1025-1030. doi:10.1016/j.dental.2024.05.006
[3] Niu Y, Wang B, Zhao Y, Zhang J, Shao B. Highly Sensitive and High-Throughput Method for the Analysis of Bisphenol Analogues and Their Halogenated Derivatives in Breast Milk. J Agric Food Chem. 2017;65(48):10452-10463. doi:10.1021/acs.jafc.7b04394
[4] Chen G, Tang C, Tan J, et al. Multi-residue determination of bisphenol analogues in organism tissues by ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2022;1682:463489. doi:10.1016/j.chroma.2022.463489
You may like
See also
Lastest Price from pyridine-3-sulfonyl chloride manufacturers

US $41.00/KG2025-04-21
- CAS:
- 16133-25-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100000
US $0.00-0.00/KG2025-04-04
- CAS:
- 16133-25-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton