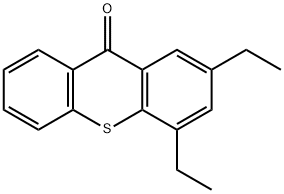
2,4-Diethyl-9H-thioxanthen-9-one - Reaction / Application on Synthetic Works
2,4-Diethyl-9H-thioxanthen-9-one is an important organic intermediate to synthetize substituted thioxanthen products.
The following example is about its application on the synthesis of 2,4-bis(1-bromoethyl)thioxanthene-9-one [1].

Into 300 mL of ethyl acetate dissolved with 26.8 g of 2,4-diethylthioxanthene-9-one (100 mmol; produced by Wako Pure Chemical Industries, Ltd.), 19.6 g of N-bromosuccinimide (110 mmol; produced by Wako Pure Chemical Industries, Ltd.) and 1.54 g of 2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile) (5.0 mmol; produced by Wako Pure Chemical Industries, Ltd.) were added, and the resulting solution was stirred at 45° C. for 30 minutes. Then, after the reaction solution was once cooled to room temperature, 19.6 g of N-bromosuccinimide (110 mmol; produced by Wako Pure Chemical Industries, Ltd.) and 1.54 g of 2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile) (5.0 mmol; produced by Wako Pure Chemical Industries, Ltd.) were added to the reaction solution, and the resulting solution was stirred at 45° C. for 1 hour. After completion of the reaction, methanol was added to the reaction solution, which was cooled to 5° C. and solvent was removed from the resulting crystal to obtain 28.9 g of 2,4-bis(1-bromoethyl)thioxanthene-9-one (pale yellow powder, yield: 68%).
The following example is about its application on the synthesis of an edg-7 receptor[2].

2,4-Diethyl-10,10-dioxo-10H-10λ6-thioxanthen-9-one Hydrogen peroxide (3.0 mL, 29.0 mmol) was added 1 mL at a time to a refluxing solution of 2,4-diethyl-thioxanthen-9-one (0.504 g, 1.88 mmol) in acetic acid (~10 mL) and allowed to stir for 2 h. The reaction was cooled to room temperature and allowed to stand 18 h. The reaction was filtered and the resulting yellow, highly viscous liquid was washed with dichloromethane and methanol, then reduced in vacuo. 2,4-Diethyl-10,10-dioxo-10H-10λ6-thioxanthen-9-one (0.381 g, 67% yield) was obtained as a yellow solid after recrystallization from ethanol and was identified on the basis of NMR spectral analysis.
The following example is about its application on the synthesis of cationic photoinitiators [3].

10.0g (0. 03731moles) of 2,4-diethylthioxanthone (DETX) were dissolved in 630 ml of a mixture of acetonitrile and water (75percent acetonitrile, 25percent water). Gentle heating was required to dissolve the DETX (45°C). 81.79g of Ceric ammonium nitrate (0. 1492moles) were added in one batch. The reaction was followed by TLC. The reaction mixture was stirred for 45minutes. At this stage TLC indicated that the reaction was complete. The reaction mixture was allowed to cool to room temperature and 400ml of water was then added. The mixture was extracted with 1000ml of diethyl ether. The ether layers were combined and dried with magnesium sulphate, and the ether was removed on a rotary evaporator to yield the product. At this stage the product still contained some inorganic residue. The product was therefore re-dissolved in diethyl ether, washed with water and dried with magnesium sulphate. The ether was then removed on a rotary evaporator to yield the product. Product is a yellow solid, yield not recorded.
The following example is about its application on the synthesis of diphenyl(5,7-diethylthioxanthene-9-one-2-yl)sulfonium trifluoromethanesulfonate [4].

To 320ml of dichloromethane were dissolved 20.2g (0.1mol) of diphenylsulfoxide and 26.8g (0.1mol) of 2,4-diethylthioxanthene-9-one, and 28.2g (0.1mol) of trifluoromethanesulfonic anhydride was added dropwise thereto at -70 to -60°C, followed by gradually warming to room temperature and reacting with stirring for 4 hours. After completion of the reaction, the obtained reaction solution was washed with water (160ml×4 times) and concentrated under reduced pressure. The obtained crude product was purified by column chromatography to obtain 38.6g of objective substance as yellow glassy substance (yield: 64 percent).
References
1. Fujifilm Wako Pure Chemical Corporation. Sakai N, Yanaba K. Acid- and radical-generating agent and method for generating acid and radical. US10451967[P], 2019, B2, Location in patent: Page/Page column 90
2. Shankar G, Solow-Cordero D, Spencer JV, Gluchowski C. Methods of treating conditions associated with an edg-7 receptor. US2004/167165[P], 2004, A1
3. Sun Chemical Corporation. Herlihy SL. Novel fused ring compounds, and their use as cationic photoinitiators. WO2003/72567[P], 2003, A1,Location in patent: Page/Page column 15
4. Wako Pure Chemical Industries, Ltd. Heterocycle-bearing onium salts. EP1481973[P], 2004, A1, Location in patent: Page 3
You may like
See also
Lastest Price from 2,4-Diethyl-9H-thioxanthen-9-one manufacturers

US $1.10/g2025-09-13
- CAS:
- 82799-44-8
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min

US $0.00/kg2025-09-02
- CAS:
- 82799-44-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons