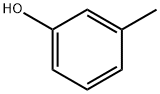
Synthesis and toxicity of m-Cresol
m-Cresol is one of the isomeric compounds of methylphenol, including o-cresol and p-cresol. m-Cresol can be used as a chemical intermediate and is widely used in the fields of medicine and pesticides. For example, when m-cresol and xylenol are mixed, they have antibacterial effects and can fight against the pathogens of crown gall and olive knot disease on fruit trees, ornamental trees, shade trees, ornamental woody shrubs and vines, and can control the genetic/physiological disease of apples - knot disease. m-Cresol can also be used in industries such as dyes, perfumes, resins, plasticizers, films and chemical inhibitors.
Synthesis
The synthesis process of m-Cresol includes steps such as salt formation, diazotization, hydrolysis, neutralization and water washing. It also provides easily accessible main raw materials and auxiliary raw materials.
(1) Salt formation
In a salt-forming pot with a cooling jacket, add 3100L of water while stirring, slowly add 1000L of concentrated sulfuric acid until the temperature of the liquid does not exceed 60°C, then cool to below 40°C, add 713Kg of m-toluidine to react, and generate m-toluidine sulfuric acid aqueous solution.
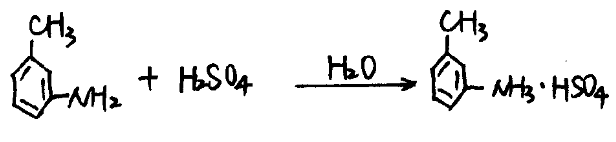
(2) Diazotization
In a diazotization pot with a cooling device, add the m-toluidine sulfate aqueous solution to the salt-forming pot, cool to -5°C, slowly add about 1200L of 40% sodium nitrite solution to react, generate m-toluidine diazonium salt, stop adding until the m-toluidine diazonium salt turns blue. Test with potassium iodide starch paper, if the m-toluidine diazonium salt is excessive, remove the excess sodium nitrite with aminosulfonic acid, and then add a certain amount of dilution water to react and complete the reaction.
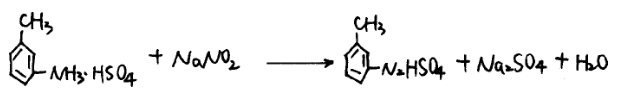
(3) Hydrolysis
Add 1800L of water, 480L of concentrated sulfuric acid and 3600L of benzene to an enameled hydrolysis pot with a heating jacket, heat to 70°C, slowly add diazonium salt of m-toluidine, reflux for 1 hour after addition, cool to about 40°C, and discharge.
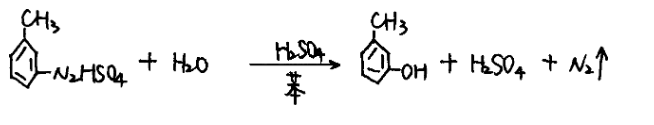
(4) Neutralization and water washing
Place the hydrolysis liquid in a conical pot and let it stand for stratification, remove the waste acid in the lower layer, neutralize the sulfuric acid remaining in the upper benzene layer with a soda ash solution, wash with water, let it stand for stratification, and remove the waste water in the lower layer. The material obtained from the above reaction is separated, crudely distilled, and rectified to obtain the finished product of m-cresol, with a content of more than 99.5% and a yield of 87%.
Toxicity
m-Cresol is toxic in skin contact, manifested by intense burning, loss of sensation, wrinkling, blanching, and softening. Gangrene may occur. It is also an eye irritant, which may cause pain, conjunctival swelling, and corneal damage. Ingestion causes a burning sensation, which may cause vomiting. Acute exposure by all routes may cause muscle weakness, gastrointestinal disorders, severe depression, and collapse. The main effects are on the central nervous system and pulmonary edema, and damage to the spleen and pancreas may also occur.
In addition, in short-term studies in rats and mice, 30,000 ppm of o-cresol, m-cresol, p-cresol, or a m-cresol/p-cresol mixture in the diet caused increased liver and kidney weights, impaired liver function, bone marrow cellularity, irritation of the gastrointestinal tract and nasal epithelium, and atrophy of female reproductive organs. In mice, 0.5% p-cresol caused loss of pigmentation, but neither m-cresol nor o-cresol caused loss of pigmentation. Developmental toxicity was found in studies with m-cresol, o-cresol, and p-cresol, but only at maternal toxic levels. No skin tumors were found in mice exposed to m-cresol, o-cresol, or p-cresol for 12 weeks.
References:
[1] JIAYING ZHU. Separation of m-cresol and p-cresol by NaZSM-5 with different Si/Al ratios.[J]. Environmental Technology, 2024. DOI:10.1080/09593330.2023.2231616.[2] ANDERSEN A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol.[J]. International Journal of Toxicology, 2006, 25 Suppl 1. DOI:10.1080/10915810600716653.
Related articles And Qustion
See also
Lastest Price from m-Cresol manufacturers

US $0.00/kg2025-03-07
- CAS:
- 108-39-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $10.00/KG2024-10-11
- CAS:
- 108-39-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton