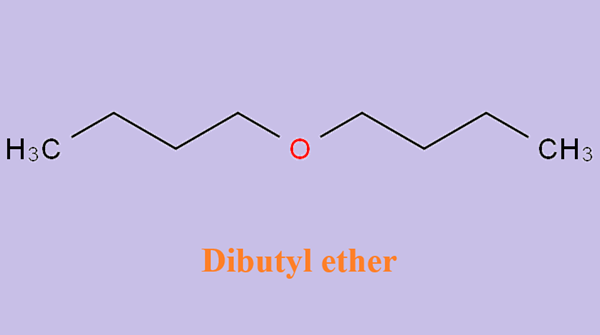
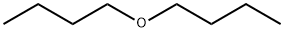Application of dibutyl ether in biofuels
Dibutyl ether (DBE) is liquid under atmospheric conditions and is immiscible with water. In the petrochemical industry, it is an excellent solvent for many natural and synthetic resins, fats, oils and organic acids. In addition, DBE is also a promising biofuel because of its high cetane number (~ 100) and high volumetric energy density (31.6 MJ/L). It can be used directly in compression ignition engines or blended with other conventional or renewable fuels.
Studies have shown that the volumetric energy densities of dibutyl ether (DBE), dimethyl ether (DME) and diethyl ether (DEE) are 31.6, 19.03 and 26.9 MJ/L, respectively. Therefore, DBE has a considerable advantage over small molecule ethers in terms of energy density. Several engine studies have highlighted the potential advantages of DBE as a diesel substitute or diesel additive, such as better ignitability, improved atomization, less soot and less unburned hydrocarbons.
A few studies have focused on the basic combustion characteristics of DBE. The ignition delay time of DBE/O2/Ar dilute mixtures at 1100–1570 K, 1.2–4 bar, and 0.5–1 equivalence ratio was measured, and the low-pressure (30 Torr) flame morphology of DBE under fuel-lean (ϕ = 0.8) and fuel-rich (ϕ = 1.5) conditions was studied by coupling with an electron ionization molecular beam mass spectrometry (EI-MBMS) system. Nearly 50 species with relatively high concentrations of n-butyraldehyde were detected and n-butyraldehyde was identified as an important fuel-specific intermediate in DBE flames. The smoke reduction capability of DBE was later studied by mixing it with methyl decanoate, and laser-induced incandescence (LII) measurements were performed in laminar co-flow diffusion flames. Through reaction path analysis, they showed that DBE can suppress propargyl recombination and ultimately the growth of aromatics to larger PAHs. Recently, speciation measurements were performed in the range of 400 – 1100 K and ϕ = 1 using an atmospheric pressure plug flow reactor coupled with EI-MBMS and two jet stirred reactors (JSRs) coupled with gas chromatography or tunable synchronous vacuum UV photoionization MBMS. The high reactivity of DBE and the presence of two NTC regions were confirmed.
However, the oxidation and pyrolysis kinetics of DBE are still unclear, especially at high pressures. We experimentally investigate the chemical kinetics of DBE in three domains: (a) ignition delay times at T = 550–650 K, P = 10, 20, 40 bar, ϕ = 0.5, 1, measured in a fast compressor; (b) ignition delay times at T = 900–1300 K, P = 20, 40 bar, ϕ = 0.5, 1, measured in a shock tube; and (c) laser-based carbon monoxide speciation measurements in a shock tube during the pyrolysis and oxidation of DBE at T = 1100–1400 K, P = 20 bar. The pressure-time curves measured in the RCM experiments show a distinctive 3-stage and 4-stage ignition behavior, mainly under fuel-lean conditions. The experimental data are compared with the predictions of two state-of-the-art chemical kinetic models for DBE. A sensitivity analysis is performed to identify key reactions that may account for the discrepancies between experiments and simulations. It was found that the decomposition rate of DBE may need to be revisited to improve the oxidation and pyrolysis predictions of DBE kinetic models.
References:
[1] KHAIYOM HAKIMOV . Ignition delay time and speciation of dibutyl ether at high pressures[J]. Combustion and Flame, 2021, 223: 1-540. DOI:10.1016/j.combustflame.2020.09.028.Related articles And Qustion
See also
Lastest Price from Dibutyl ether manufacturers

US $0.00/KG2025-04-21
- CAS:
- 142-96-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00-10.00/KG2025-04-15
- CAS:
- 142-96-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg