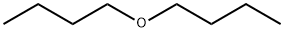
Dibutyl ether
- Product NameDibutyl ether
- CAS142-96-1
- CBNumberCB2775063
- MFC8H18O
- MW130.23
- EINECS205-575-3
- MDL NumberMFCD00009461
- MOL File142-96-1.mol
- MSDS FileSDS
Chemical Properties
| Melting point | -98 °C (lit.) |
| Boiling point | 142-143 °C (lit.) |
| Density | 0.764 g/mL at 25 °C (lit.) |
| vapor density | 4.48 (vs air) |
| vapor pressure | 4.8 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point | 77 °F |
| storage temp. | Store at <= 20°C. |
| solubility | H2O: soluble0.113g/L at 20°C |
| form | Liquid |
| color | Clear colorless |
| PH | 5.2 (0.2g/l, H2O, 20℃) |
| Relative polarity | 1.7 |
| Odor | Mild, ether-like. |
| PH Range | 5.2 |
| explosive limit | 0.9-8.5%(V) |
| Relative density, gas (air=1) | 0.764 g/mL at 25 °C |
| Water Solubility | 0.03 g/100 mL (20 ºC) |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H226-H315-H319-H335-H412 | |||||||||
| Precautionary statements | P210-P233-P240-P273-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10-36/37/38-52/53 | |||||||||
| Safety Statements | 61-24/25 | |||||||||
| RIDADR | UN 1149 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | EK5425000 | |||||||||
| Autoignition Temperature | 365 °F | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29091990 | |||||||||
| Hazardous Substances Data | 142-96-1(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 7.4 g/kg (Smyth) | |||||||||
| NFPA 704: |
|


