m-Cresol- application
m-Cresol is a methylphenol compound, which is naturally occurring or synthetic, and can be used as cosmetic biocides/preservatives in cosmetics. m-Cresol function as both a preservative and fragrance ingredient.
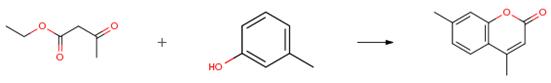
m-Cresol can be reacted with formaldehyde to form phenolic resins as the basic polymer for use in polymeric coatings that may be safely used as the food-contact surface of articles intended for use in producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food.
m-Cresol can be used to produce phenolic resins and used safely as the food-contact surface of molded articles intended for repeated use in contact with nonacid food (pH above 5.0). The phenolic resins are produced from one or more selected phenols and one or more selected aldehydes (phenols: p-tert-amylphenol, p-tert-butylphenol, m-, o-, and p-Cresol, p-octylphenol, phenol, and o- and p-phenylethylphenol mixture produced when phenol is made to react with styrene in the presence of sulfuric acid catalyst; aldehydes: acetaldehyde, formaldehyde, and paraldehyde). The extracted phenol should not exceed 0.005 mg per square inch of food-contact surface and, in accordance with good manufacturing practice, finished molded articles containing phenolic resins shall be thoroughly cleansed prior to first use in contact with food (21CFR177.2410).
Hamaguchi and Tsutsui noted that m-Cresol is used as a topical dental antiseptic, and Wappler et al. reported that m-Cresol is used in insulin preparations, and is suspected to be a trigger of malignant hyperthermia. FDA determined that m-Cresol has not been established as safe and effective in topical antifungal drug products (21CFR310.545).
m-Cresol,is a biomarker for phenol exposure. Bieniek assayed o-Cresol (found only in test subjects) and p-Cresol from human urine by thinlayer chromatograph (TLC). o-Cresol (76.9 mg/L) and p-Cresol (58.6 mg/L) were detected in the urine of workers employed in the distillation of the high temperature phenolic fraction of tar (carbolic oil) and in nonexposed workers at 68.1 g/L (o-Cresol) and 25.7 mg/L (p-Cresol). Bieniek (1997) assayed m-Cresol, o- Cresol and p-Cresol from human urine by GC-MS. All subjects were male smokers exposed mainly to phenol, Cresols, xylenols, and other phenolic derivatives while employed in the distillation of carbolic oil.
In addition, m-Cresol is an important organic intermediate (building block) to synthetize substituted aryl products. [1] For instance, it can be applied in the synthesis of aryl tosylates and mesylates; in the synthesis of mucohalic acids and in vinylation of phenols and naphthols with acetylene.
References
1.Vahabi V, Hatamjafari F. Microwave assisted convenient one-pot synthesis of coumarin derivatives via Pechmann condensation catalyzed by FeF3 under solvent-free conditions and antimicrobial activities of the products[J]. Molecules, 2014,19(9):13093-13103.
2.Lei X, Jalla A, Abou S, Mhd A, Stafford JM, Cao B. Chromatography-Free and Eco-Friendly Synthesis of Aryl Tosylates and Mesylates[J]. Synthesis, 2015, 47(17): 2578 - 2585
3.Blazecka PG, Zhang J. Preparation of substituted butenolides via palladium-free etherification and amination of masked mucohalic acids. US2005/59831[P], 2005, A1, Location in patent: Page/Page column 7-8
4.Trofimov OK, Vysotskaya G. Nucleophilic addition to acetylenes in superbasic catalytic systems: XVIII. Vinylation of phenols and naphthols with acetylene[J]. Russian Journal of Organic Chemistry, 2015, 51(2):188 – 19
Related articles And Qustion
See also
Lastest Price from m-Cresol manufacturers

US $0.00/kg2025-03-07
- CAS:
- 108-39-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $10.00/KG2024-10-11
- CAS:
- 108-39-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton