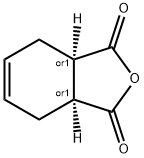
Synthesis of cis-1,2,3,6-Tetrahydrophthalic anhydride
Introduction
Tetrahydrophthalic anhydride is one of the downstream products of maleic anhydride. It is a high-performance new type of organic anhydride epoxy resin curing agent used for the preparation of alkyd resins and unsaturated resins. It has the advantages of improving the adhesion, elasticity, gloss, and water resistance of coatings. As a plasticizer, tetrahydrophthalic anhydride can improve the cold and heat resistance of PVC, and is non-toxic, so it is widely used in the electronic and electrical industry, aerospace field. In addition, this product can also be used as a raw material for synthesizing surfactants and pharmaceutical pesticide products. Cis-1,2,3,6-Tetrahydrophthalic anhydride is one of them.(Figure1)

In the course of a synthetic problem it became necessary to prepare some cis-1,2,3,6-hexahydrophthalicacid. The syntheses of this acid described in the literature are those of Baeyer, Willstster and Jaquet, and DIels and Alder. Because of slow reaction speeds and repeated recrystallizations these procedures are time-consuming and laborious. All the repeated recrystallizations, as well as the vacuum concentration of the final product, were eliminated. In addition to this, the time required for the reaction of the maleic anhydride and butadiene was greatly reduced, as was also the time needed for the reduction of the cis-1,2,3,6- trahydrophthalic anhydride. This article lists the initial synthesis methods and the optimized current synthesis methods.
The initial synthesis method of cis-1,2,3,6-Tetrahydrophthalic Anhydride
Ninety-eight grams (1 mole) of finely ground maleic anhydride was partly dissolved and partly suspended in 150ml of benzene. After this suspension had been cooled to 5℃, 54 g. (1 mole) of liquid butadiene was added. The mixture was then heated in an autoclave to 115℃. At this temperature heating was discontinued, but within fifteen minutes the temperature had spontaneously risen to 145℃and the pressure to 11 atmospheres. The reaction mixture was left to stand overnight in the autoclave.
The crude product was freed from benzene and was then extracted with ether by use of a Soxhlet extractor. A white crystalline mass of pure cis-1,2,3,6-tetrahydrophthalic anhydride formed in the ether during the extraction, and more precipitated upon cooling to room temperature.The unreacted maleic anhydride remained in the ether solution but could be recovered by cooling the solution to 0℃. The product melted at 103-104℃. The yield was 137 g., or 90.3% of the theoretical amount. [1]
Improved synthesis method of cis-1,2,3,6-Tetrahydrophthalic Anhydride [2]
1. Cis-1,2,3,6-Tetrahydrophthalic anhydride was synthesized via continuous method. The effects of solvent, reaction temperature, pressure, reactant mole ratio and residence time on the conversion of maleic anhydride and the yield of cis-1,2,3,6-tetrahydrophthalic anhydride was investigated and the optimal reaction conditions were obtained. Using tetrahydrofuran as a solvent, at maleic anhydride concentration of 10%, polymerization inhibitor of 0.1% (the quality of maleic anhydride), temperature of 140℃, pressure of 1.5Mpa,residence time of 0.8h and a molar ratio of butadiene to maleic anhydride of 1.6, the conversion of maleic anhydride was 98.93%, the content of cis-1,2,3,6-tetrahydrophthalic anhydride in the product solution amounted to 96.86% and the content of butadiene in the tail gas was 12.5%. The product solution was concentrated and cooled to get solid cis-1,2,3,6-tetrahydrophthalic anhydride (THPA), the purity of the solid was 99.07% and the yield was 67.13%.
2. Cis-1,2,3,6-Tetrahydrophthalic anhydride was also synthesized via batch method using tetrahydrofuran asa solvent. They tested the effects of solution concentration, temperature, reactant mole ratio, and residence time on the reaction. At maleic anhydride concentration of 9%, temperature of 130℃, residence time of 60min and amolar ratio of butadiene to maleic anhydride of 1.3, the pressure less than1.0Mpa, the conversion of maleic anhydride was 99.63%, the content of cis-1,2,3,6-tetrahydrophthalic anhydride in the product solution amounted to 98.33% and the content of butadiene in the tail gas was 7.51%.
3. Meanwhile, the isomerization of cis-1,2,3,6-tetrahydrophthalic anhydride catalyzed by P2O5 to a mixture of tetrahydrophthalic anhydride for the production of liquid tetrahydrophthalic anhydride was also investigated. Cis-1,2,3,6-tetrahydrophthalic anhydride was called △4-THPA in its isomers. The isomerization process had two steps. Firstly, △4-THPA isomerized to △3-THPA; Secondly,△3-THPA isomerized to △1-THPA. The isomerization process was sensitive to temperature and the amount of catalyst can influence the process too. In the tested catalysts, P2O5 was the most effective. The freezing point of the mixture dropped from 99-101℃(freezing point of △4-THPA) down to below -25℃ and then rose up to 74℃(freezing point of △1-THPA).
References
[1] Jenkins, E. F., & Costello, E. J. (1946). An Improved Synthesis of cis-Δ4-Tetrahydrophthalic Anhydride and cis-Hexahydrophthalic Acid. Journal of the American Chemical Society, 68(12), 2733–2733. doi:10.1021/ja01216a512
[2] Fan, C. (2009). Synthesis?of 1,2,3,6-Tetrahydrophthalic anhydride and its isomerizaton. Beijing University of Chemical Technology. DOI:10.7666/d.y1558175.(MA Thesis)
See also
Lastest Price from cis-1,2,3,6-Tetrahydrophthalic anhydride manufacturers

US $120.00/kg2025-04-21
- CAS:
- 935-79-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000
US $10.00/KG2025-04-21
- CAS:
- 935-79-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt