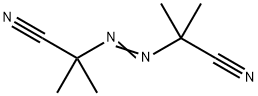
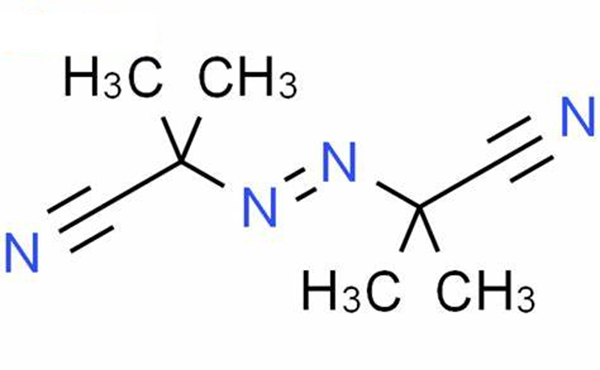
Uses of Azobisisobutyronitrile (AIBN)
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator [1]. It's soluble in a wide variety of organic solvents, including alcohol-based solvents. Insoluble in water and denser than water. Moderately toxic by ingestion. Readily ignited by sparks or flames. Burns intensely and persistently. Toxic oxides of nitrogen produced during combustion. Used as a catalyst, in vinyl polymerizations and a blowing agent for plastics [2].
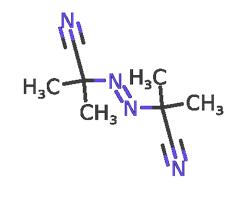
Azobisisobutyronitrile (AIBN) is an azo-compound and is widely used as a free radical initiator. This compound has labile carbon-nitrogen covalent bond which undergoes homolytic scission under thermal, chemical or photochemical conditions producing free radicals. They are useful in many reactions like halogenation, polymerisation of vinyl monomers, grafting reactions, curing of rubbers and unsaturated polymers and cross-linking of polyolefins. AIBN can be used as an initiator in the synthesis of highly cross-linked Poly(divinylbenzene) (PDVB) polymers. It also can be used as an initiator in the polymerization process of 2-hydroxyethyl methacrylate (HEMA).
AIBN is produced from acetone cyanohydrin and hydrazine, then followed by oxidation [3]:
2(CH3)2C(CN)OH + N2H4 → [(CH3)2C(CN)]2N2H2 + 2 H2O
[(CH3)2C(CN)]2N2H2 + Cl2 → [(CH3)2C(CN)]2N2 + 2 HCl
Related azo compounds behave similarly, such as 1,1′-azobis(cyclohexanecarbonitrile). Water-soluble azo initiators are also available.
In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals:
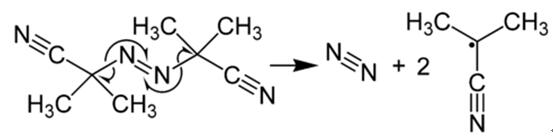
These radicals can initiate free-radical polymerizations and other radical-induced reactions. For instance, a mixture of styrene and maleic anhydride in toluene will react if heated, forming the copolymer upon addition of AIBN. Another example of a radical reaction that can be initiated by AIBN is the anti-Markovnikov hydrohalogenation of alkenes.
It is often used as a foamer in plastics and rubber and as a radical initiator. In most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals: These radicals can initiate free-radical polymerizations and other radical-induced reactions.
AIBN is safer to use than benzoyl peroxide (another radical initiator) because the risk of explosion is far less. However, it is still considered as an explosive compound, decomposing above 65 °C. A respirator dust mask, protective gloves and safety glasses are recommended. Pyrolysis of AIBN without a trap for the formed 2-cyanopropyl radicals results in the formation of tetramethylsuccinonitrile, which is highly toxic.
References
[1] https://en.wikipedia.org/wiki/Azobisisobutyronitrile
[2] https://pubchem.ncbi.nlm.nih.gov/compound/2_2_-Azobis_2-methylpropionitrile
[3] Schirmann, Jean-Pierre; Bourdauducq, Paul. "Hydrazine". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_177.
Related articles And Qustion
See also
Lastest Price from 2,2'-Azobis(2-methylpropionitrile) manufacturers

US $100.00-350.00/kg2025-04-17
- CAS:
- 78-67-1
- Min. Order:
- 1000kg
- Purity:
- 0.98
- Supply Ability:
- 5000 Metric Ton/Metric Tons per Year

US $10.00/kg2025-03-07
- CAS:
- 78-67-1
- Min. Order:
- 20kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON