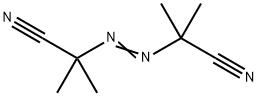
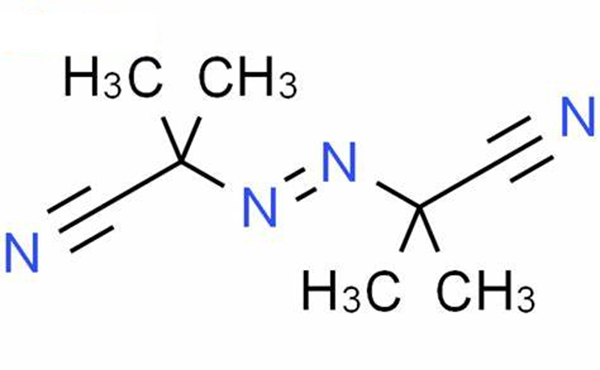
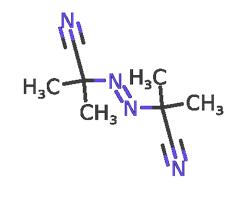
What is 2,2'-Azobis(2-methylpropionitrile)?
An initiator is a substance that can be decomposed to generate primary free radicals under the action of heat, light, radiation, etc., and can initiate the polymerization of monomers. The initiator plays a very important role in free radical polymerization[1]. The type and amount of initiator have a great relationship with the polymerization reaction rate and the molecular weight of the polymer. At a certain temperature, the polymerization rate of a certain monomer depends largely on the decomposition rate of the initiator. Therefore, it is of practical significance to study and determine the decomposition rate of the initiator to control the polymerization reaction[2]. There are many types of initiators with different properties, but according to their chemical composition, they can be roughly divided into two categories: peroxides and azos. According to the generation of free radicals, they can be divided into thermally initiated (including light and thermal radiation) systems and redox systems. Among azo compounds, 2,2'-Azobis(2-methylpropionitrile) is one of the most common initiators[3].
2,2'-Azobis(2-methylpropionitrile) is used as an initiator in polymer radical polymerization because its molecules can easily undergo split reactions and form molecules with high activation energy[4]. 2,2'-Azobis(2-methylpropionitrile) (AIBN) is the most commonly used azo initiator. Its characteristic is that the decomposition reaction is relatively stable, only one kind of free radical is generated, and basically no induced decomposition occurs, so it is often used in the kinetics research of free radical polymerization[5]. In addition, it is relatively stable and safe to store and use. Like all azo compounds, AIBN is also toxic and cannot be used in the synthesis of polymers related to medical and food packaging. Because its decomposition reaction produces stoichiometric nitrogen, it can often be easily liberated by measuring its fraction[6]. The volume of nitrogen is used to determine the kinetic data such as its decomposition activation energy and frequency factor. In some cases, the polymer can be foamed by the released nitrogen gas. Its decomposition temperature is 50~70 ℃, and its decomposition activation energy is 129kJ/mol, which belongs to a low activity initiator[7].
References
[1] Biswajit Ray, Broja M. Mandal. Dispersion Polymerization of Acrylamide: Part II. 2,2′-2,2'-Azobis(2-methylpropionitrile) Initiator[J]. Journal of Polymer Science Part A Polymer Chemistry, 1999, 37(4):493-499.
[2] Jay K. Kochi, David M. Mog. Decomposition of 2,2'-Azobis(2-methylpropionitrile) in the Presence of Cupric Salts. Oxidation of α-Cyanoalkyl Radicals[J]. Journal of the American Chemical Society, 1965, 87(3).
[3] Xu H, Liu PT, Li YH. Copper-Mediated Direct Aryl C-H Cyanation with 2,2'-Azobis(2-methylpropionitrile) via a Free-Radical Pathway[J]. Organic Letters, 2013, 15(13):3354-3357.
[4] Saroj Sharma, A.K. Srivastava. Synthesis and characterization of copolymers of limonene with styrene initiated by 2,2'-Azobis(2-methylpropionitrile)[J]. 40(9):2235-2240.
[5] Liu, Lianghui, Wang, Zikuan, Fu, Xuefeng. ChemInform Abstract: 2,2'-Azobis(2-methylpropionitrile) Initiated Aerobic Oxidative Transformation of Amines: Coupling of Primary Amines and Cyanation of Tertiary Amines.[J]. Cheminform, 44(14):no-no.
You may like
Related articles And Qustion
See also
Lastest Price from 2,2'-Azobis(2-methylpropionitrile) manufacturers

US $100.00-350.00/kg2025-04-17
- CAS:
- 78-67-1
- Min. Order:
- 1000kg
- Purity:
- 0.98
- Supply Ability:
- 5000 Metric Ton/Metric Tons per Year

US $10.00/kg2025-03-07
- CAS:
- 78-67-1
- Min. Order:
- 20kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON