What is Malonic acid?
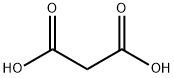
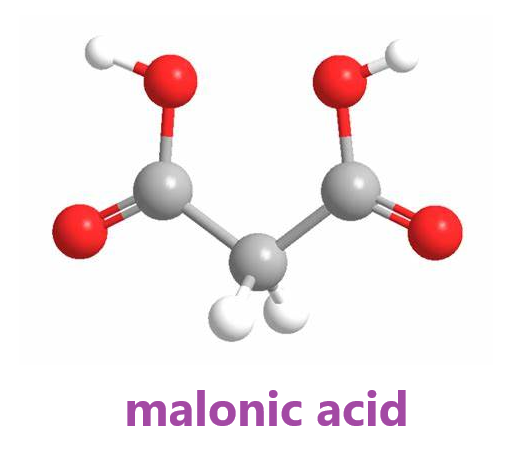
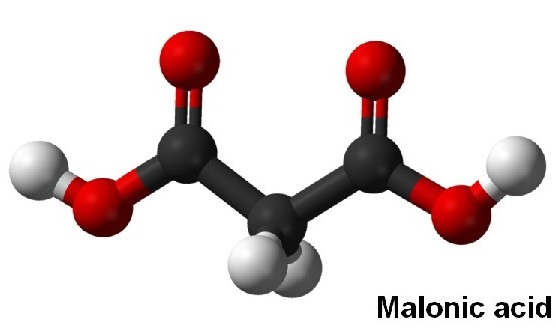
Malonic acid, also known as Propanedioic acid, Methane dicarbonic acid or Carboxyacetic acid, is an organic acid. Mainly used in perfumes, pharmaceutical intermediates, adhesives, resin additives, electroplating polishing agents, etc[1]. Used in the pharmaceutical industry to produce barbituric acid, vitamin B1, vitamin B2, vitamin B6, etc[2]. In agriculture, malonic acid is an intermediate of the fungicide rice pestin and an intermediate of the plant growth regulator indyl ester. In addition, it can also be used in the production of adhesives and resin additives, and can also be used as a surface treatment agent for leather and aluminum products[3]. When malonic acid is used as an aluminum surface treatment agent, since only water and carbon dioxide are generated during thermal decomposition, there is no pollution problem. In this regard, it has great advantages compared with acid-based treatment agents such as formic acid used in the past. Malonic acid is also used as an additive for electroplating, and also used as an electroplating polishing agent, welding additive, and the like[4].
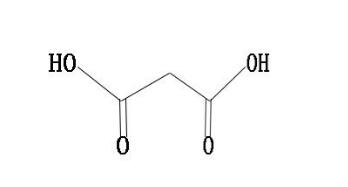
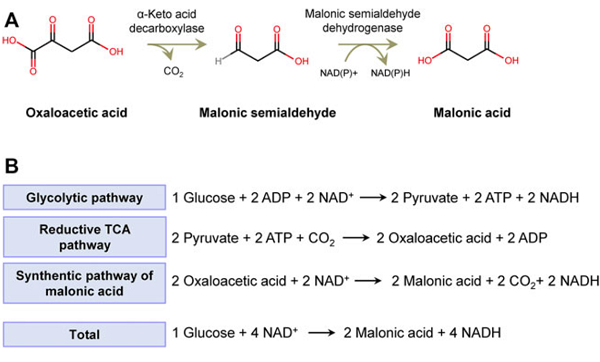
Malonic acid has both carboxyl and active methylene functional groups in its molecular structure, so it can participate in various chemical reactions and is a very important intermediate for organic synthesis. Since the methylene group in the malonic acid molecule is activated by two carboxyl groups, various types of reactions can occur[5]. It can be reacted with a base, and can also undergo esterification, acid halide, amidation and reduction reactions. Malonic acid can show all the properties of fatty dicarboxylic acids, but has fundamentally different chemical properties from its homologs[6]. Dehydration and decarboxylation can occur to produce α-β unsaturated monocarboxylic acids. Bromine substituted methine can obtain intermediates such as monobromomalonic acid and dibromomalonic acid. Malonic acid loses carbon dioxide when heated and generates acetic acid. By using this property to decarboxylate substituted malonic acid, various carboxylic acids can be synthesized[7].
References
[1] Edward P, Faryniarz, Raymond J, et al. Cosmetic compositions containing salts of malonic acid[J]. 2003.
[2] Bigeleisen, Jacob, Friedman, Lewis. C13 Isotope Effect in the Decarboxylation of Malonic Acid[J]. Journal of Chemical Physics, 17(10):998.
[3] George A. Hall. The Kinetics of the Decomposition of Malonic Acid in Aqueous Solution[J]. Journal of the American Chemical Society, 2002, 71(8):2691-2693.
[4] Faryniarz J R, Johnson A W, Cheney M C, et al. Cosmetic compositions with hydroxy amine salts of malonic acid[J]. 2005.
[5] Mcconnell H M, Heller C, Cole T, et al. Radiation Damage in Organic Crystals. I. CH(COOH)2 in Malonic Acid1[J]. 1960, 82(4).
Related articles And Qustion
See also
Lastest Price from Malonic acid manufacturers

US $120.00/kg2025-08-02
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 60 tons

US $1.00/kg2025-04-21
- CAS:
- 141-82-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt