Reactions and metabolism of malonic acid
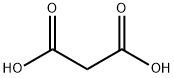
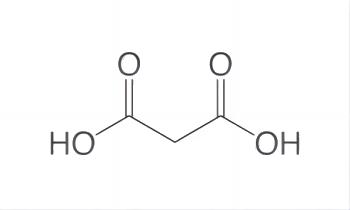
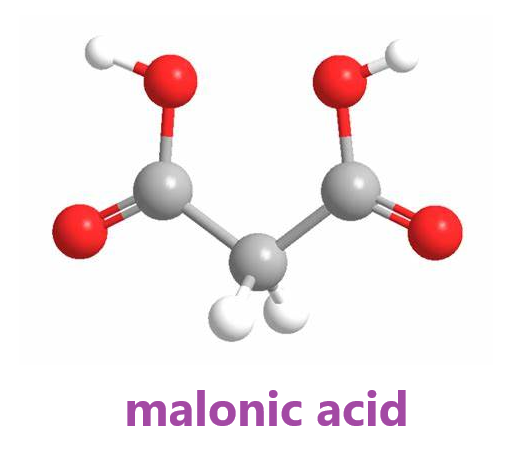
Malonic acid, with the chemical formula CH2(CO2H)2, is a simple dicarboxylic acid that is widely used in organic synthesis and chemical reactions. In addition, malonic acid injection improves the survival rate of rats subjected to acute hypoxia. It is speculated that endogenous malonic acid plays an important role in enhancing tissue resistance to hypoxia.
Reactions
Malonic acid has been detected in various atmospheric environments, both in the gas phase and in aqueous aerosols. It is a prototype compound with a carboxylic acid group that can undergo keto-enol tautomerization and can therefore undergo autocatalytic keto-enol tautomerization. As an extension of the acid-catalyzed dihydrogen transfer mechanism, this process is not only of fundamental significance but may also help explain the tautomerization of malonic acid observed in solution and aerosol particles. We demonstrate through quantum chemical calculations that the keto-enol tautomerization of malonic acid can be catalyzed by the two tautomers of malonic acid itself. This autocatalytic process has a relatively low barrier (Gibbs energy of about 13 kcal/mol in gas phase and 20 kcal/mol in aqueous phase) and involves the concerted transfer of two protons between the substrate and the carboxylic acid functional group of the malonic acid catalyst. This mechanism is expected to compete with the proton relay mechanism currently used to explain the tautomerization of malonic acid in aqueous media.
When malonic acid, ceric sulfate and potassium bromate are dissolved in dilute sulfuric acid, an oscillatory chemical reaction occurs, manifested by oscillations in light absorption caused by ceric ions (317 mµ) and oscillations in the rate of carbon dioxide evolution.
Metabolism
It has been shown that malonic acid decomposes under the irradiation of ultrasound, and the main gaseous products are CO, CO2 and H2. Carbon monoxide was only detected in the ultrasonic decomposition method. Acetic acid and formic acid were confirmed as intermediates in this reaction.
The metabolism of malonic acid by bovine sperm was studied by using malonic acid-C14 acid. Factors affecting malonate metabolism were determined by production of C14O2 from malonate-1-C14 acid; metabolic pathways in malonate metabolism were investigated by chemical degradation of malonate-2-C14 acid, paper chromatography, autoradiography, and isolated intermediates. C14O2 production from malonate-1-C14 acid was determined at pH 5.3, 5.6, 6.0, and 6.5. Maximum production of C14O2 was obtained at pH 6.0. No significant increase in radioactive carbon dioxide production was observed after cold shock or versene treatment, suggesting that osmotic pressure is not limiting malonate metabolism.
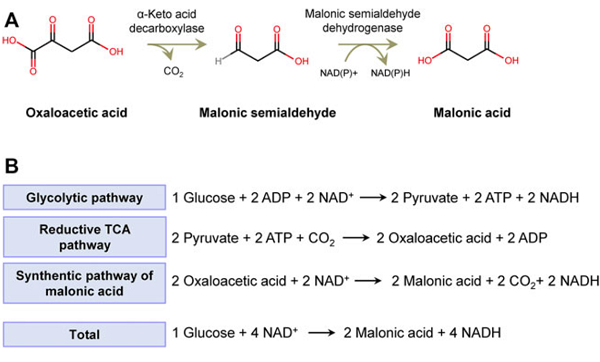
Glutamate and aspartate were identified as metabolites of malonate by co-chromatography. Specific activity of a single carbon of glutamate yielded the expected pattern of glutamate biosynthesis, i.e., transamination of the citric acid cycle intermediate α-ketoglutarate. The data suggest that malonate is decarboxylated to form acetate, which is metabolized via the citric acid cycle.
References:
[1] CATHERINE C. R. SUTTON Gabriel da S Chia Yang Lim. Self-catalyzed keto-enol tautomerization of malonic acid[J]. International Journal of Quantum Chemistry, 2019, 120 5. DOI:10.1002/qua.26114.
[2] V V DAVYDOV A V R. [Protective effect of malonic acid in hypoxic hypoxia].[J]. Fiziologicheskiĭ zhurnal, 1991, 37 5: 111-112.
[3] J.L. FLEEGER R. J F. Metabolism of Bovine Semen. XIII. Malonic Acid Metabolism by Bovine Spermatozoa1, 2[J]. Journal of Dairy Science, 1964, 47 5: Pages 535-538. DOI:10.3168/jds.S0022-0302(64)88705-1.
[4] DEGN H. Effect of Bromine Derivatives of Malonic Acid on the Oscillating Reaction of Malonic Acid, Cerium Ions and Bromate[J]. Nature, 1967, 213 5076: 589-590. DOI:10.1038/213589a0.
Related articles And Qustion
See also
Lastest Price from Malonic acid manufacturers

US $120.00/kg2025-08-02
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 60 tons

US $1.00/kg2025-04-21
- CAS:
- 141-82-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt