Polarity and Solubility of Malonic Acid
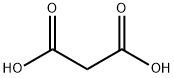
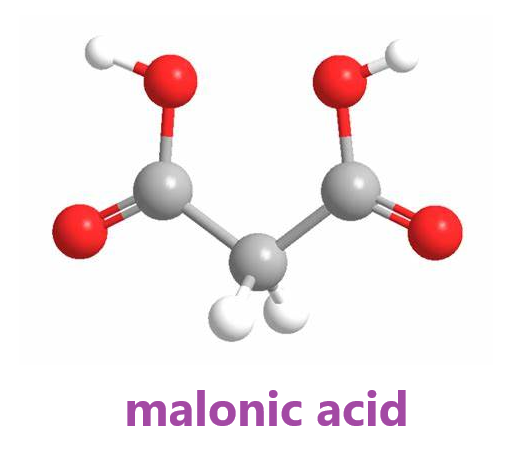
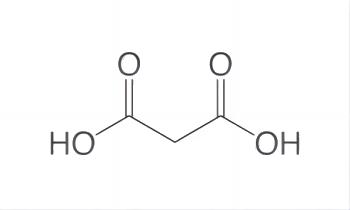
Malonic acid is a dicarboxylic acid belonging to the family of carboxylic acids. A dicarboxylic acid contains two carboxylic acid functional groups. Usually, a dicarboxylic acid exhibits the same chemical behavior as monocarboxylic acids. This naturally occurs in certain fruits. It is a useful organic compound with various benefits. Its IUPAC name is propanedioic acid. It should not be confused with malic or maleic acid.
It is an organic compound naturally found in some fruits. Fruits produced in organic farming have greater concentrations of malonic acid than those generated from conventional farming practices. It is often found in some citrus fruits and vegetables. It is a component of food items, it is present in animals, including humans.
Polarity
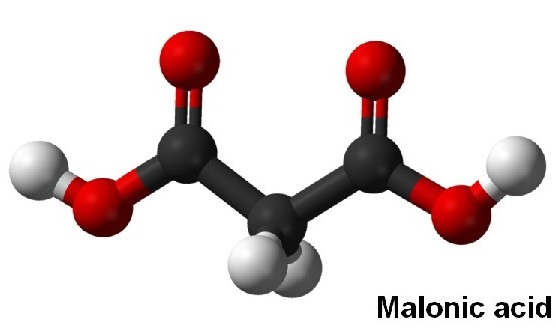
Carboxyl group is polar as there is a large difference in the electronegativity values of oxygen and hydrogen. Malonic acid has two carboxyl groups and only three carbon atoms, which has little effect on polarity, so the malonic acid molecule is polar.
Solubility
Sample of malonic acid was tested with water, methyl alcohol, and hexane. Malonic acid was soluble in water because both malonic acid and water are polar. It took 25 seconds for malonic acid to dissolve in water. Malonic acid was soluble in methyl alcohol because malonic acid is polar and methyl alcohol is intermediately polar, allowing malonic acid to dissolve in the methanol in 15 seconds. Malonic acid was insoluble in hexane because hexane is nonpolar while malonic acid is polar.
You may like
Related articles And Qustion
See also
Lastest Price from Malonic acid manufacturers

US $120.00/kg2025-08-02
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 60 tons

US $1.00/kg2025-04-21
- CAS:
- 141-82-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt