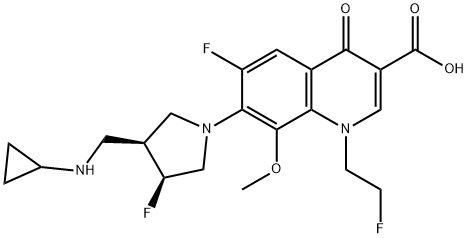
The synthesis method of Lascufloxacin hydrochloride
Overview
Lascufloxacin hydrochloride (AM-1977) is a novel 8-methoxy fluoroquinolone antibacterial agent with a unique pharmacophore at the 1st and 7th positions of the quinoline nucleus developed by Kyorin Pharmaceutical Co., Ltd. (Tokyo, Japan). It is suitable for treating infections caused by Staphylococcus, Streptococcus, Pneumococcus, Moraxella (Branhamella) catarrhalis, Klebsiella, Enterobacter, Haemophilus influenzae, Legionella pneumophila, Prevotella and Mycoplasma pneumoniae that are sensitive to this drug[1–2].
Synthetic method

Researchers at Kyorin Pharmaceuticals devised a kilogram scale synthesis of the drug. The molecular structure consists of a fluoroquinoline core appended with a syn-disubstituted fluoropyrrolidine fragment. To prepare the fluoropyrrolidine fragment, the authors employed a linear route starting from amino ester 14 [3]. Cbz protection of compound 14 followed by Claisen condensation afforded pyrrolidone 15 in 75% yield—asymmetric hydrogenation using a ruthenium catalyst with (S)-BINAP as the ligand provided trans-pyrrolidine 16. Saponification of the ester followed by coupling with cyclopropylamine furnished crystalline amide 18, isolated in 60% yield from 15. The amide was then reduced to the corresponding amine with borane THF and isolated as its hydrochloride salt. Benzyl protection gave trialkyl amine 20, treated with perfluoro-1-octane sulfonyl fluoride (POSF), to deliver chiral fluoride 21 with inversion of stereochemistry. Benzyl deprotection and salt formation produced fluoropyrrolidine fragment 22 as a crystalline solid.
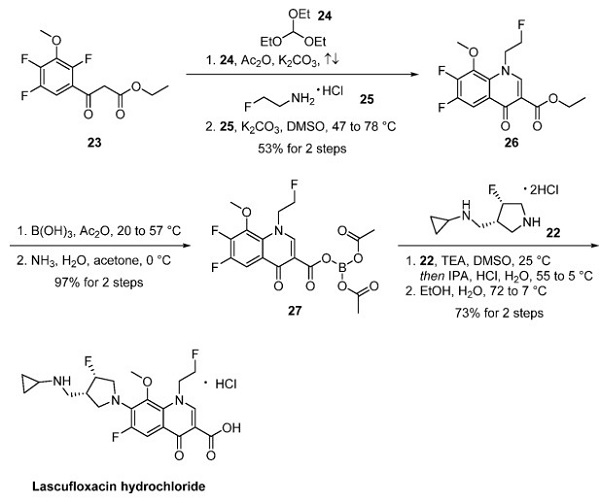
The fluoroquinolone core was constructed from acetoacetate 23. Condensation of compound 23 with triethyl orthoformate (24) followed by treatment with 2-fluoroethylamine hydrochloride (25) afforded quinolone 26. Interestingly, the ethyl ester was converted to borate complex 27 via treatment with boric acid and acetic anhydride, which was noted to provide improved solubility and enhanced reactivity in the subsequent SNAr reaction—finally, substitution of 27 with fluoropyrrolidine 22 provided lascufloxacin.
References
[1] R Thakare. “Lascufloxacin hydrochloride to treat bacterial infection.” Drugs of today 56 6 (2020): 365–376.
[2] Ryuta Kishii, Masaya Takei, Yuko Yamaguchi. “In Vitro Activities and Spectrum of the Novel Fluoroquinolone Lascufloxacin (KRP-AM1977).” Antimicrobial Agents and Chemotherapy (2017).
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.