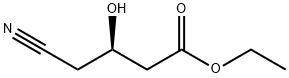
What is Ethyl (R)-(-)-4-cyano-3-hydroxybutyate?
Hydroxyglutaryl coenzyme A (HMG-CoA) reductase inhibitors are a new class of lipid-lowering drugs, and they have a market share of more than 10 billion US dollars in the current pharmaceutical field[1]. They can selectively inhibit the rate-limiting enzyme HMG-CoA reductase in cholesterol synthesis in the liver, reduce liver lipoprotein production, and increase the expression of low-density lipoprotein (LDL) cholesterol receptors, thereby reducing plasma cholesterol levels[2]. They can also significantly reduce very low-density lipoprotein (VLDL) and triglycerides, and increase anti-atherosclerotic high-density protein (HDL), preventing atherosclerosis and coronary heart disease.

Ethyl (R)-(-)-4-cyano-3-hydroxybutyate is an important intermediate for the synthesis of HMG-CoA reductase inhibitors[3]. If it is used as a raw material, synthetic atorvastatin is mainly used for the treatment of hypercholesterolemia and mixed hyperlipidemia, coronary heart disease and stroke. Sales amounted to $ 7.8 billion in 2008. Therefore, how to reduce the synthesis cost and study the new method for the preparation of ethyl R-(-)-4-cyano-3-hydroxybutyrate have been the focus of attention at home and abroad[4]. In the reported synthesis.The main methods are:
Starting from chiral 3-hydroxy-γ-butyrolactone, Ethyl (R)-(-)-4-cyano-3-hydroxybutyate was prepared through ring opening, esterification and cyanation[5].
Racemic epichlorohydrin was used as a raw material to produce ethyl R-(-)-4-cyano-3-hydroxybutyrate using nitrilase.
A chiral epichlorohydrin was used as a raw material to undergo cyanide ring opening and nitrile hydrolysis, and then esterify to synthesize ethyl R-(-)-4-cyano-3-hydroxybutyrate[6].
A strain was obtained by screening by Chartrain, etc., and the ethyl 4-chloroacetoacetate could be biotransformed into ethyl S-4-cyano-3-hydroxybutyrate, and then cyanated to Ethyl (R)-(-)-4-cyano-3-hydroxybutyate[7].
The fermentation method is not efficient and is still in the laboratory research stage; the product obtained by the asymmetric synthesis method is not high in optical purity; the chiral source conversion method requires multi-step synthesis, the yield is low, and there are many by-products.
References
[1] Ming-Jia Yang, Xiang-Jing Wang, Zhong-Yi Yang. Bioconversion of ethyl (R)-4-cyano-3-hydroxybutyate into (R)-ethyl-3-hydroxyglutarate via an indirect pathway byRhodococcus boritolerans[J]. 34(5):901-905.
[2] Hua-Ping Dong, Zhi-Qiang Liu, Yu-Guo Zheng. Novel biosynthesis of (R)-ethyl-3-hydroxyglutarate with (R)-enantioselective hydrolysis of racemic ethyl 4-cyano-3-hydroxybutyate byRhodococcus erythropolis[J]. 87(4):1335-1345.
[3] Yao P, Li J, Yuan J, et al. Enzymatic Synthesis of a Key Intermediate for Rosuvastatin by Nitrilase‐Catalyzed Hydrolysis of Ethyl (R)‐4‐Cyano‐3‐hydroxybutyate at High Substrate Concentration[J]. 2015, 7(2):271-275.
[4] Burk, Mark J, Desantis, Grace, Morgan, Brian, et al. Processes for making [R]-ethyl 4-cyano-3 hydroxybutyric acid[J].
[5] Jiang, Chengjun, Hong, Huabin. ChemInform Abstract: A New Practical Synthesis of Ethyl (R)-(-)-4-Cyano-3-hydroxybutyrate (VII) from (S)-3-Chloro-1,2-propanediol (I).[J]. Cheminform, 44(1):no-no.
See also
Lastest Price from Ethyl (R)-(-)-4-cyano-3-hydroxybutyate manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 141942-85-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton

US $50.00-30.00/kg2025-03-07
- CAS:
- 141942-85-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20Tons