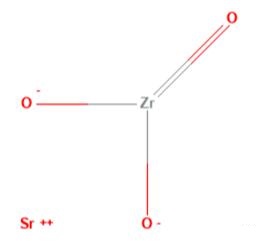
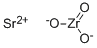What is Strontium zirconate?
Strontium zirconate (STRONTIUM ZIRCONATE, STRONTIUM (II) ZIRCONATE; zirconate(zro32-), strontium(1:1); STRONTIUM (II) ZIRCONATE; STRONTIUM ZIRCONATE; STRONTIUM ZIRCONIUM OXIDE; strontium zirconium trioxide; Strontium zirconium oxide, 99.3%metalsbasis; SrZrO3) as mixed metal oxides with different structural characteristics can be widely applied in the field of electronic ceramics and refractory materials of high melting temperatures, and also can be applied in fuel cells and hydrogen sensors due to their proton conductivity at high temperatures [1]. The performance of strontium zirconate would be related to powder purity, crystal structure and size, which mainly depends on synthesis procedure. The synthesis routes for obtaining strontium with similar purity, homogeneity and chemical composition can fall into solid and wet methods, including conventional solid state reaction, combustion process, co-precipitation, hydrothermal methods, the Pechini process and citrate complexation [2].
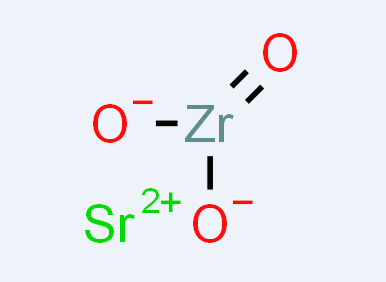
The conventional solid state reaction of of SrCO3 and ZrO2 is a typical solid route that require high temperature calcination process with intermittent grinding and prolonged duration of calcination for promoting atom diffusion and forming strontium zirconate powder (1200°C/48 h) [3]. However, the obtained strontium zirconate might display unsatisfactory powder characteristics, such as inhomogeneity, impurity contamination, non-uniform size and distribution. Recently, by using solid state reaction and spark plasma sintering technique, Valeanu groups have successfully synthesized strontium zirconate with sub-micron average crystallite size and without intermediate calcination and grinding steps [4].
Wet routes, such as pyrolysis, combustion process, co-precipitation, hydrothermal methods have been proposed. By using strontium zirconyl oxalate as precursor, the fine strontium zirconate powders could be prepared through three-step, including dehydration, decomposition of oxalate and decomposition of carbonate [5]. The chemical co-precipitation method with advantages in simplicity and ease of handling is also used in the synthesis of ultrafine strontium zirconate powders under tunable pH conditions. In co-precipitation method, to remove contamination of the final product, other chemical species such as citric acid as chelating agents could be added for synthesis oxide materials with better purity and homogeneity [6]. For example, Potdar and his coworkers have developed a simple chemical coprecipitation route to synthesize a single phase molecular precursor strontium zirconate at room temperature by exchange reaction between freshly prepared aqueous solutions of sodium zirconyl oxalate and equimolar strontium nitrate. After pyrolysis at 900°C/5 h in air, strontium zirconate with submicron sized monophasic orthorhombic structure were obtained [6]. By the solution combustion synthesis process, the mixture of fuels, such as glycine, urea and ammonium nitrate, and corresponding metal nitrates as oxidizer was dissolved in minimum water, and underwent boiling, frothing, foaming, igniting to burn for obtaining strontium zirconate [7]. In other work, Koshy groups have developed a modified combustion process for preparing nanocrystals of strontium zirconium oxide, in which citric acid and ammonia as complexing agent and oxidant fuel respectively, were used to replace polyvinyl alcohol and urea to get a single phase strontium zirconium as nanocrystals in a single step combustion [8]. In addition, Athawale and his coworkers have prepared strontium zirconium with nanocrystalline and orthorhombic (space group Pnma) structure by a rapid hydrothermal method using nitrate salts of strontium and zirconium as precursors in time period as less as half-an-hour [9].
In conclusion, according to different synthesis methods, strontium zirconate with different crystal structure and size could be obtained for meeting the various application requirements.
References
[1] Thomas, J. K., Kumar, H. P., Pazhani, R., Solomon, S., Jose, R., & Koshy, J. (2007). Synthesis of strontium zirconate as nanocrystals through a single step combustion process. Materials Letters, 61(7), 1592-1595.
[2] de Oliveira Lima, J. R., Ghani, Y. A., da Silva, R. B., Batista, F. M. C., Bini, R. A., Varanda, L. C., & de Oliveira, J. E. (2012). Strontium zirconate heterogeneous catalyst for biodiesel production: Synthesis, characterization and catalytic activity evaluation. Applied Catalysis A: General, 445, 76-82.
[3] Keler, E. K., & Kuznetsov, A. K. (1961). Properties of barium zirconate. Zh. Prikland. Khimii, 34(10), 2146.
[4] Popescu, B., Enache, S., Ghica, C., & Valeanu, M. (2011). Solid-state synthesis and spark plasma sintering of SrZrO3 ceramics. Journal of Alloys and Compounds, 509(22), 6395-6399.
[5] Cavalcante, L. S., Simoes, A. Z., Sczancoski, J. C., Longo, V. M., Erlo, R., Escote, M. T., ... & Varela, J. A. (2007). SrZrO3 powders obtained by chemical method: synthesis, characterization and optical absorption behaviour. Solid State Sciences, 9(11), 1020-1027.
[6] Potdar, H. S., Deshpande, S. B., Patil, A. J., Deshpande, A. S., Khollam, Y. B., & Date, S. K. (2000). Preparation and characterization of strontium zirconate (SrZrO3) fine powders. Materials Chemistry and Physics, 65(2), 178-185.