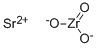Strontium Zirconate-Application
Strontium zirconate was most actively investigated concerning the possibility of its use in magnetohydrodynamic plants. The components of these plants experience the effect of high-temperature (up to 2700°C) high-speed (above 1000 msec) gas flow and electromagnetic fields and are in contact with alkaline additive, oxidizing reactants, and zirconium dioxide electrodes[1]. Strontium zirconate proved to be suitable for such service conditions.
Strontium zirconate has been the subject of several investigations because of its technological applications. For instance, Strontium zirconate can be used in the electronic industry as insulators, and its refractory properties are of interest in high-temperature applications. In nuclear safety studies, Strontium zirconate plays an important role as it is formed in the UOz fuel by reaction between the fission products in the fuel matrix, and during core-concrete interactions between the fission products and the oxidized zircaloy cladding. The thermodynamic properties, the structural variants and phase transitions are thus of special interest as they influence the behaviour of hazardous fission products.
SrZrO3 (SZO) has been the subject of investigations because of its technological applications. For instance, the wide band gap and high dielectric constant of SZO can be used in the electronic industry, and its refractory properties are of interest in high-temperature applications [2, 3]. Moreover, SZO thin films may be regarded as one of the most promising candidate materials used in optoelectronic devices because of the wide band gap and transparent to visible light.
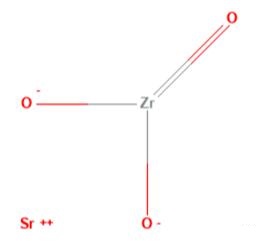
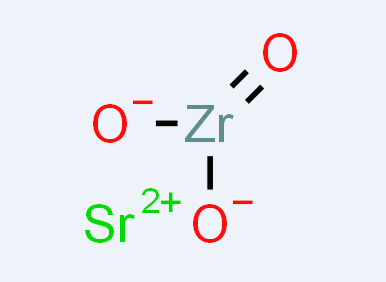
Fig 1. Chemical structure formula of Strontium Zirconate
Strontium zirconate (SZO), with chemical formula SrZrO3, is a complex oxide with a number of useful properties for device applications. Its features include high temperature proton conductivity, a large dielectric constant, resistance switching , and ferroelectricity in artificial superlattices. SZO crystallizes in the perovskite (ABO3) structure, a class of compounds that is currently of significant research interest due to the emergence of novel interface phenomena. Native points defects are known to play an important role in oxides and in many cases dominate the electronic and optical properties. For example, native defects commonly act as carrier-compensation centers, introduce optically active states in the band gap, and are sometimes invoked as sources of free carriers[4-8].
References
[1] High-Temperature Materials for Magnetohydrodynamic Power Plants [in Russian], Nauka, Moscow
[2] D. DeLigny, P. Richet. Phys. Rev. B, 53 (1996), pp. 3013-3022.
[3] E.A. Slonimskaysa, A.V. Belyakov. Glass Ceram., 58 (1–2) (2001), pp. 54-56.
[4] T. Yajima, H. Suzuki, T. Yogo, H. Iwahara.Solid State Ion., 51 (1992), p. 101.
[5] M.-H. Lin, M.-C. Wu, C.-Y. Huang, C.-H. Lin, T.-Y. TsengJ. Phys. D: Appl. Phys., 43 (2010), p. 295-404.