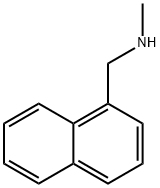
1-Methyl-aminomethyl naphthalene-Application
1-Methyl-aminomethyl naphthalene has been used as reagent for the determination of isocyanates in air by UV or fluorescence detection. 1-Methyl-aminomethyl naphthalene has been used in the preparation of key intermediate required for the synthesis of terbinafine.
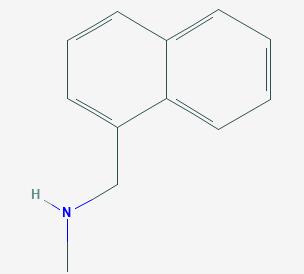
Fig 1. Chemical structure formula and three-dimensional structure of 1-Methyl-aminomethyl naphthalene
Condensation is carried out using chloro-t-butane and ethyl chloride as raw materials in the presence of aluminum trichloride. Then in the presence of potassium hydroxide, it is heated at a high temperature in dimethyl sulfoxide to eliminate two molecules of hydrogen chloride. The obtained material is reacted with ethyl magnesium bromide at room temperature, and then reacted with acrolein to form 6,6-dimethyl-1-heptene-4-yn-3-ol.Finally bromination gives cis-trans-1-bromo-6,6-dimethyl-2-heptene-4-yne. The obtained product and N-methyl-1-naphthylmethylamine are condensed under the action of sodium carbonate to obtain terbinafine and its cis isomer. The two can be separated by recrystallization from isopropanol, or by column chromatography.
Terbinafine is an allylamine antifungal drug, which inhibits squalene epoxidase during the process of ergosterol synthesis in fungal cells, and causes squalene to accumulate in cells to play a bactericidal role. The sensitivity of human cells to this product is one ten thousandth of that of fungi. This product has a broad-spectrum antifungal effect, bactericidal effect on skin fungi, and antibacterial effect on Candida albicans. It is suitable for skin and nail infections caused by superficial fungi, such as Trichophyton, Microsporum canis, Epidermophyton floccus, and other infections such as ringworm, jock itch, athlete's foot, onychomycosis, and candida albicans.
In the preparation process, terbinafine hydrochloride is mainly used. The main synthesis route in the existing synthesis method of terbinafine is: first, 1-chloromethylnaphthalene and monomethylamine are reacted to obtain N -Methyl-1-naphthylmethylamine; then, N-methyl-1-naphthylmethylamine is reacted with 1-chloro-6,6-dimethyl-2-heptene-4-yne to give terbinafine[1-4].
References
[1] Julia A. Balfour, Diana Faulds. Terbinafine[J]. Drugs, 1995, 43(2):259-284.
[2] N. S. Ryder, I. Leitner. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species[J]. Medical Mycology, 2009.
[3] Balfour J A, Faulds D. Terbinafine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses.[J]. 1992, 43(2):259-84.
[4] N.S. RYDER. The mechanism of action of terbinafine[J]. Clinical & Experimental Dermatology, 1989, 14(2).
Related articles And Qustion
Lastest Price from 1-Methyl-aminomethyl naphthalene manufacturers

US $0.00/KG2025-04-21
- CAS:
- 14489-75-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 14489-75-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt