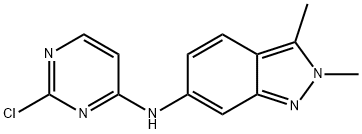
How to prepare N-(2-Chloropyrimidin-4-YL)-2,3-dimethyl-2H-indazol-6-amine?
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine can be prepared from 2-ethylaniline by nitration, cyclization and methylation reactions. It is mainly used as a pharmaceutical chemical raw material for the preparation of the drug molecule pazopanib hydrochloride.

Synthesis of N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine
At room temperature, 2,4-dichloropyrimidine (13.9 g) was added to a solution of 2,3-dimethyl-2H-indazol-6-amine (5.00 g) and NaHCO3 (10.4 g, 124 mmol) in anhydrous ethanol (100 mL) under stirring. The resulting reaction mixture was then stirred at 75°C for about 4 hours. The reaction progress was monitored by TLC spot plate. After the reaction was completed, the reaction mixture was concentrated and filtered under vacuum to remove insoluble precipitates in the reaction system. The reaction mixture is washed with ethyl acetate and the filtrate is concentrated under vacuum. The residue is separated and purified by slurrying with ethyl acetate to obtain the target product molecule N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine.