An analytical study of empagliflozin in the risk of gout
Introduction of Empagliflozin
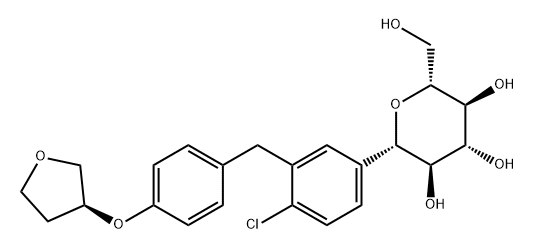
Empagliflozin is an FDA-approved antidiabetic drug in the sodium-glucose cotransporter protein (SGLT-2) class of diabetes medications that is used in adults with type 2 diabetes, and in patients with type 2 diabetes with heart and blood vessel disease to reduce the risk of stroke, heart attack, or death. Empagliflozin is also used in adult patients with heart failure to reduce the risk of hospitalisation and death due to heart and blood vessel disease. Empagliflozin can be used as a single medication or in combination with other antidiabetic products.
Mechanism of Action
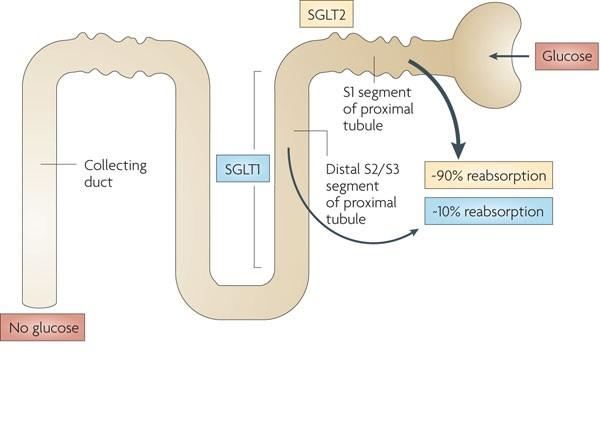
Empagliflozin acts by inhibiting sodium-glucose cotransporter protein-2 (SGLT-2) found in the proximal tubules of the kidney. Through SGLT2 inhibition, empagliflozin decreases renal reabsorption of glucose and increases urinary excretion of glucose. The hypoglycaemic effect of the drug is independent of insulin. In patients with type 2 diabetes mellitus, 10 mg of empagliflozin adds approximately 64 grams per day and 25 mg adds 78 grams per day. Rosiglitazar reduces sodium and volume load, causing intravascular constriction through its diuretic and natriuretic properties. Additionally, it has been shown to reduce blood glucose levels in patients with type 2 diabetes. Additionally, Empagliflozin is associated with weight loss and may lower blood pressure without increasing heart rate.
Risk of gout
Patients with type 2 diabetes mellitus (T2D) frequently develop hyperuricaemia and are associated with an increased risk of gout. Engeletin may reduce serum urate levels by promoting urate excretion. By evaluating the association between treatment with empagliflozin in routine clinical practice and the risk of developing gout. After excluding patients with a history of gout or gout medication, we identified 102,262 1:1 propensity score-matched pairs of adult T2D patients initiating treatment with either empagliflozin or a DPP4 inhibitor (DPP4i) (Cohort 1) and 131,216 pairs initiating treatment with either empagliflozin or a GLP-1 receptor agonist (GLP-1RA) (Cohort 2). We estimated pooled HR and RD/1,000 PY and their 95% CIs, adjusted for 141 baseline covariates, and performed stratified analyses within multiple subgroups.
During a treatment period with a mean follow-up of approximately 8 months, compared with patients initiating treatment with DPP4i [HR, 0.69 (0.60, 0.79); RD -2.27 (-3.08, -1.46)] or GLP-1RA [HR 0.83 (0.73, 0.94); RD -0.99 (-1.66, -0.32)], patients initiating treatment with empagliflozin treatment had a lower risk of gout. The results were consistent across gender, age, body mass index, hyperlipidemia, chronic renal failure subgroups, and diuretic use (Table). In clinical practice, initiation of empagliflozin was associated with a lower risk of developing gout compared with initiation of DPP4i or GLP-1RA.
References:
[1] H. TESFAYE. 870-P: Empagliflozin and the Risk of Gout—Analysis from the Empagliflozin Comparative Effectiveness and Safety (EMPRISE) Study[J]. Diabetes, 2023. DOI:10.2337/db23-870-p.
[2] C. ALVAREZ. 873-P: Empagliflozin Compliance and Influencing Factors in Patients with Heart Failure and Diabetes[J]. Diabetes, 2023, 1 1. DOI:10.2337/db23-873-p.
You may like
Related articles And Qustion
Lastest Price from Empagliflozin manufacturers

US $0.00-0.00/KG2025-12-05
- CAS:
- 864070-44-0
- Min. Order:
- 1KG
- Purity:
- 99.5%min
- Supply Ability:
- 100KGS
US $0.00/kg2025-10-11
- CAS:
- 864070-44-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS