Pharmacological effects of empagliflozin
Overview
Empagliflozin is a type 2 sodium glucose cotransporter (SGLT-2, sodium-dependent glucose cotransporter2) inhibitor jointly developed by Boehringer Ingelheim and Eli Lilly[1]. SGLT-2 inhibitor is a new type of hypoglycemic drug, mainly by inhibiting the expression of SGLT-2, reducing the renal reabsorption of glucose, increasing the excretion of glucose in the urine, thereby reducing the level of plasma glucose, and its hypoglycemic effect is independent of in beta cell function and insulin resistance. Empagliflozin is used for the treatment of adult patients with type 2 diabetes who cannot achieve adequate glycemic control with diet and exercise to improve glycemic control. It should not be used to treat patients with type 1 diabetes, patients with elevated blood or urine ketones (diabetic ketoacidosis), patients with severe renal impairment, end-stage renal disease, and patients on dialysis.

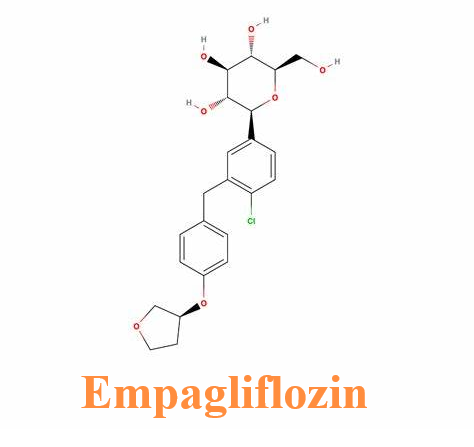
Picture 1 Empagliflozin powders
Pharmacological effects of empagliflozin
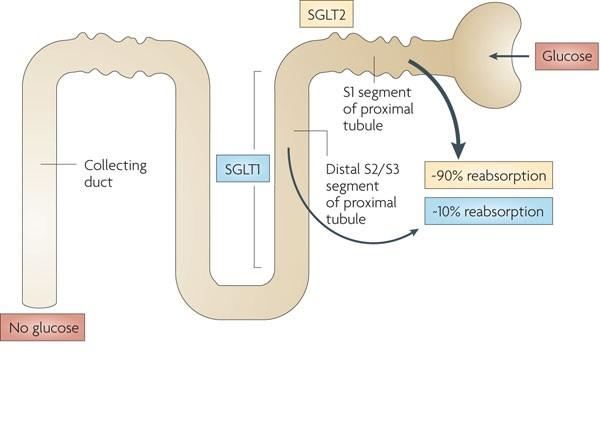
Under the physiological environment, the kidney enters the circulatory system by filtration and reabsorption of glucose to maintain the blood glucose balance in the body, while the glucose enters the blood through the renal tubular lumen, which must be coordinated by the glucose co-transporter. SGLT2 acts as a glucose co-transporter. A form of cotransporter that blocks the reabsorption of glucose in the proximal convoluted tubule, reduces glucose reabsorption in the kidneys, and increases excretion of glucose in the urine, thereby reducing plasma glucose levels. And the hypoglycemic effect does not depend on β-cell function and is not affected by insulin resistance.
Adverse reactions to empagliflozin
Empagliflozin had a low incidence of adverse reactions. Compared with the placebo group, the incidence of common adverse reactions was 2%, including urinary tract infection, female reproductive system fungal infection, upper respiratory tract infection, polyuria, dyslipidemia (dose-related low-density lipoprotein increased), arthralgia, fungal infection of male reproductive system, hypoglycemia (the incidence of hypoglycemia increased when empagliflozin was combined with insulin or sulfonylurea hypoglycemic drugs), nausea. Other adverse reactions less than 2% were common in thirst (polydipsia, polydipsia), hypovolemia, and renal dysfunction.
Preparation of empagliflozin
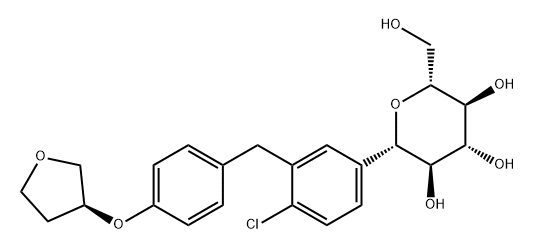
Using 5-bromo-2-chlorobenzoic acid as raw material, the intermediate (S)-4-bromo-1-chloro-2-(4-tetrahydro) was obtained through acid chloride, Friedel-Crafts acylation, nucleophilic substitution and reduction. : furan-3-yloxy-benzyl)benzene, the intermediate is condensed with 2,3,4,6-tetra-O-trimethylsilyl-D-glucopyranosic acid 1,5-lactone , etherification and demethylation to obtain empagliflozin, the total yield is 29.8%, and the purity is 99.13%. The diabetes drug empagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor, which is Boehringer On August 1, 2014, the FDA officially approved Boehringer Ingelheim and Eli Lilly's Jardiance® (empagliflozin) for the treatment of Type 2 diabetes to improve glycemic control in adult patients. empagliflozin is the third FDA-approved SGLT-2 inhibitor class. Two other SGLT-2 inhibitors, Johnson & Johnson's Invokana® (canagliflozin), AstraZeneca and Bristol-Myers Squibb's Farxiga® (dapagliflozin), have been released in November 2013, respectively. January and 2Chemicalbook014 received FDA approval. Empagliflozin's new drug application to the FDA can be described as twists and turns. In March 2014, a new drug application for empagliflozin submitted by the Boehringer-Lilly Diabetes Alliance was rejected by the FDA due to a large particle contamination incident at a Boehringer empagliflozin production plant. In June 2014, the FDA withdrew the previously issued warning letter after confirming that the quality management and compliance system of the Boehringer drug production plant was acceptable based on the inspection summary in March and fully reviewing the materials submitted by Boehringer. The Boehringer-Lilly Diabetes Alliance also resubmitted its application to the FDA on June 17.
Clinical studies of empagliflozin
The FDA's approval of empagliflozin was based on the results of seven clinical trials conducted in nearly 4,500 patients with type 2 diabetes, and all patients taking empagliflozin had significantly lower HbA1c levels compared to the placebo group. The most common adverse reactions were urinary tract infections and female reproductive system infections. On March 21, 2014, the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use recommended approval of the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin for the treatment of adults with type 2 diabetes. On May 23, 2014, the European Medicines Agency (EMA) approved the marketing of empagliflozin in Europe. On June 16, 2014, the Boehringer-Lilly Diabetes Alliance released the latest data from two Phase III clinical trials of empagliflozin at the 74th American Diabetes Association Scientific Congress (ADA2014). In a 2-year study, the addition of empagliflozin or glimepiride to metformin in adults with type 2 diabetes showed that empagliflozin reduced HbA1c more than glimepiride, The weight and blood pressure treatment effects were comparable to glimepiride.
Another 52-week study of empagliflozin or placebo in addition to multiple daily insulin injections in patients with type 2 diabetes who were still unable to adequately control blood sugar levels with high-dose insulin (with or without metformin) showed results Compared with placebo, empagliflozin significantly reduced blood sugar levels and body weight, while reducing insulin doses. In both studies, the safety profile of empagliflozin was consistent with previous studies. The FDA notice states that the drug should not be used in patients with type 1 diabetes, elevated blood or urine ketones (diabetic ketoacidosis), severe kidney damage, end-stage renal disease, or dialysis patients. The most common side effects were urinary tract infections and female genital infections. Empagliflozin can cause dehydration and lead to a drop in blood pressure, which can lead to dizziness or fainting and decreased kidney function. Elderly patients, especially those with impaired renal function and diuretics use, appear to be at greater risk. FDA requires Boehringer and Eli Lilly to conduct four postmarketing studies: completion of ongoing cardiovascular outcomes trials, pediatric pharmacokinetics and pharmacodynamics trials, pediatric safety and efficacy trials, Tests (especially effects on kidney function and bone and growth).
Reference
1 Zinman B, Wanner C, Lachin J M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes[J]. New England Journal of Medicine, 2015, 373(22): 2117-2128.
You may like
Related articles And Qustion
See also
Lastest Price from Empagliflozin manufacturers

US $0.00-0.00/KG2025-12-05
- CAS:
- 864070-44-0
- Min. Order:
- 1KG
- Purity:
- 99.5%min
- Supply Ability:
- 100KGS
US $0.00/kg2025-10-11
- CAS:
- 864070-44-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS