Different preparation methods of pyruvic acid
Background
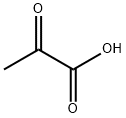
Pyruvic acid, also known as a-oxopropionic acid, is an organic compound with a chemical formula of C3H4O3 and a structure of CH3COCOOH. It is an important intermediate in the sugar metabolism of all biological cells and the mutual conversion of various substances in the body, because the molecule contains activated ketone and As a basic chemical raw material, it is widely used in various fields such as chemistry, pharmacy, food, agriculture and environmental protection, and can be prepared by various methods of chemical synthesis and biotechnology[1].

Picture 1 Pyruvic acid tablets
Physical properties
Light yellow to yellow transparent liquid. Smell of acetic acid. Has a sour taste. Natural products exist in mint and sucrose fermentation broth. The relative molecular mass is 88.06. Relative density 1.2271. Melting point 13.8 ℃. Boiling point 165°C (decomposition), 106.5°C (13.332×103Pa), 85.3°C (5.333×103Pa), 70.8°C (2.666×103Pa), 57.9°C (1.333×103Pa), 45.8°C (0.667×103Pa), 21.4°C ( 0.133×103Pa). Flash point 82 ℃. Refractive index 1.4280. Miscible with water, ethanol, ether, etc.
Chemical properties
Darkens in air. It polymerizes slowly when heated, is highly reactive, easily reacts with nitrides, aldehydes, halides, phosphides, etc., and participates in the sugar metabolism of organisms, biochemical synthesis and metabolism of colloids, amino acids, proteins, etc., and alcohol fermentation. When exerted, it is reduced to lactic acid in the muscle, re-oxidized and partially converted to glycogen at rest. Oral LD502100mg/kg for rats. Pyruvic acid is a component of the human body, which is mainly involved in the metabolism of sugar and fat in the human body, and is also one of the intermediate products of carbohydrate metabolism.
The role in metabolism
Pyruvic acid is a weakly acidic organic acid with two functional groups, carbonyl and carboxyl, in the molecule. It has the properties of α-keto acid in addition to the properties of carboxylic acid and ketone. It is the simplest α-ketone. Acid (belongs to carbonyl acid). Pyruvic acid is a tricarbon keto acid produced in the body. It is the final product of the glycolysis pathway. It is reduced to lactic acid for energy in the cytoplasm, or oxidized into the mitochondria to form acetyl CoA, which enters the tricarboxylic acid cycle and is oxidized to carbon dioxide. and water to complete the aerobic oxidation of glucose for energy supply. Pyruvic acid can also realize the mutual conversion of sugars, fats and amino acids in the body through the acetyl-CoA and tricarboxylic acid cycles. Therefore, pyruvic acid plays an important pivotal role in the metabolic connection of the three nutrients.
The anti-oxidize effect
Studies have shown that pyruvic acid can inhibit the oxidation of oxygen free radicals in mice, and as a hydrogen peroxide scavenger, it has the effect of preventing free radical damage, and it has been proved to be protective in cardiac reperfusion injury and acute renal failure. The body resists functional damage. Pyruvic acid can act as an antioxidant through two mechanisms: first, as an α-keto acid, pyruvic acid can directly inhibit hydrogen peroxide through a non-enzymatic decarbonation reaction; second, supplementation of pyruvic acid can enhance In the citric acid cycle, after the production of citric acid increases, the phosphofructokinase is inhibited, thereby entering the pentose phosphate bypass to generate reduced coenzyme II (NADPH), thereby indirectly increasing the ability of the glutathione (GSH) antioxidant system. Pyruvic acid can also increase the ratio of coenzyme I/reduced coenzyme I (NAD+/NADH) and promote the reaction of the tricarboxylic acid cycle
Preparation of pyruvic acid by dehydration and decarboxylation of tartaric acid
This method is simple and easy to implement: the mixture of tartaric acid and potassium hydrogen sulfate is distilled at 220 ° C, and the distillate is then subjected to vacuum rectification to obtain pyruvic acid. The characteristic of this method is that after adding the heat transfer oil, the reaction is carried out in a homogeneous system, which reduces the reaction temperature, reduces the degree of oxidation, and greatly improves the operability. It is suitable for continuing the reaction to generate pyruvic acid series products. The disadvantage is that the yield of pyruvic acid is low, and 5g of potassium hydrogen sulfate is needed to obtain 1g of pyruvic acid. The cost of raw materials alone is 80,000 yuan per ton, which cannot be accepted by most manufacturers because the cost is too high.
Preparation of pyruvic acid by Lactic acid oxidation
Using lactic acid as raw material, pyruvic acid is produced by one-step oxidative dehydrogenation. However, it is very difficult to directly prepare pyruvic acid from lactic acid, and appropriate catalysts must be selected according to different processes. The catalysts that can be selected are iron phosphate, tellurium molybdate, silver, vanadium and the like. Compared with the oxidative decarboxylation method of tartaric acid, this method has the advantages of low energy consumption, low pollution and high yield, and is suitable for industrial production. The disadvantage is that the cost is also high, about 60,000 yuan per ton.
Enzyme-catalyzed preparation of pyruvic acid
Using enzymes or microbial cells as catalysts to convert certain intermediate metabolites of glucose or tricarboxylic acid cycles into pyruvic acid under certain conditions is called enzymatic catalysis. The main process is to first carry out small-scale microbial culture, collect the bacteria, directly transform or embed it into an immobilized enzyme with a carrier, and then transform into pyruvic acid. The catalytic method has small investment in equipment, low energy consumption and high conversion rate, but the source of the substrate is narrow and the cost is relatively high about 50,000 yuan per ton, so its further promotion is limited.
Preparation of pyruvic acid by genetic engineering technology
The use of genetic recombination technology to construct genetically engineered bacteria with high expression of glycolate oxidase, catalase, etc., for the production of pyruvic acid. These enzymes catalyze the reaction of lactic acid with oxygen to produce pyruvic acid. The technology is to first recombine the glycolate oxidase gene and the catalase gene with a DNA vector to form recombinants, and then transfer them into host cells respectively to obtain genetically engineered yeasts with high expression of the two enzymes. - Sodium lactate solution per 100ml of humidified weight transformant is 5g, and a certain amount of penetrant is added at the same time, and oxygen is introduced at 70psig oxygen pressure under 5 atmospheres, and the conversion is stirred at 5 °C for 4 hours, and the yield of pyruvic acid is 97.7%. The substrate conversion rate of this technology is high, but the technology is difficult.
Preparation of pyruvic acid by microbial fermentation
In the process of microbial metabolism, the process of using glucose to accumulate pyruvic acid is called microbial fermentation. The research on the production of pyruvic acid by microbial fermentation has a history of 50 years, but the breeding of high-yielding strains of pyruvic acid is very difficult. Although some microorganisms can accumulate pyruvic acid, their yield cannot meet the requirements of industrialization. The real breakthrough in the production of pyruvic acid by this method was in 1988, when researchers Miyata Reiko and Yoneharazu, both of Japan's Toray Industries Co., Ltd., selected a series of strains of Saccharomyces sp. The industrialization of pyruvic acid production by fermentation is possible. In 1992, Japan began to use microbial fermentation to produce pyruvic acid. The output is 400 tons per year, and the cost is about 20,000-30,000 yuan per ton.
Compared with chemical synthesis method and enzymatic conversion method, microbial fermentation method has advantages due to wide source of raw materials, low energy consumption, less pollution and low cost. However, the disadvantage of microbial fermentation method is that the conversion rate is relatively low. This is because pyruvic acid is a key intermediate product of the glycolysis pathway. In cells, pyruvic acid, as an important intermediate metabolite, connects the central metabolic pathway of EMP and TCA. Associated with multiple branched metabolic pathways, it can be converted into a variety of fermentation products and cannot be accumulated in the body. Therefore, it needs to be cut off or weakened for further metabolism, so that it can accumulate in a large amount in cells. That is to speed up the conversion rate of glucose to pyruvic acid, weaken the flux to the TCA cycle, cut off or weaken its branch metabolic pathway, promote secretion, weaken the reuse of pyruvic acid, and finally achieve a large accumulation of pyruvic acid. In order to achieve this purpose, it is necessary to study the influencing factors of pyruvic acid production by microbial fermentation. The factors affecting the production of pyruvic acid by microbial fermentation include: strain selection, nutritional conditions, vitamin levels, oxygen supply mode, glucose concentration, etc. The most critical factors are strain selection and nutritional conditions.
Reference
1 Li Y, Chen J, Lun S Y. Biotechnological production of pyruvic acid[J]. Applied microbiology and biotechnology, 2001, 57(4): 451-459.
Related articles And Qustion
See also
Lastest Price from Pyruvic acid manufacturers
US $100.00/ASSAYS2025-04-21
- CAS:
- 127-17-3
- Min. Order:
- 1ASSAYS
- Purity:
- 98%min
- Supply Ability:
- 200TON

US $6.00/kg2025-04-21
- CAS:
- 127-17-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month