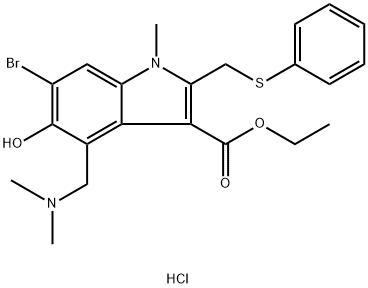
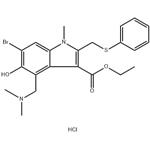
Different formulations and pharmacokinetics of Arbidol hydrochloride
Introduction of Arbidol hydrochloride
Arbidol hydrochloride is a broad-spectrum antiviral agent that targets a wide range of enveloped and non-enveloped viruses. It is used to prevent influenza virus (IFV)-induced severe pneumonia and virus-associated cytokine dysregulation. It also has a number of immunomodulatory properties, including interferon-induced and macrophage-activating effects.

Abidol is used clinically in the treatment of influenza to prevent viral entry into cells and to prevent fusion of the virus with host cell membranes. Abidol has been reported to have in vitro antiviral activity against not only influenza virus and hepatitis C virus (HCV), but also Ebola virus, COVID-19 and SARS-CoV-2.
Different formulations and pharmacokinetics
To compare the pharmacokinetic properties of four formulations (dispersible tablets, plain tablets, capsules and granules) of the broad-spectrum antiviral drug Arbidol hydrochloride in beagle dogs. A single dose of 100 mg of each of the four formulations of Arbidol hydrochloride was administered orally to the dogs, and then plasma samples were prepared by blood collection from a vein in the foreleg at various times after administration. Plasma concentrations of Arbidol hydrochloride were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The results showed that there were no significant differences in pharmacokinetic parameters, including time to peak, peak concentration, elimination half-life, area under the curve (AUC0-t) and mean retention time, between orally administered dispersible tablets of Arbidol hydrochloride, plain tablets, capsules and aqueous suspension granules.
However, in the case of the dispersible tablets, the pharmacokinetics of arbidol hydrochloride was significantly affected by the mode of administration. Compared with direct feeding, peak time [0.50 (0.13, 0.50) vs. 1.00 (0.50, 2.00)] was significantly shortened (P = 0.033) and the AUC0–48 h (8726.5 ± 2509.3 vs. 3650.8 ± 1536.9 ng h/ml) was significantly increased (P = 0.012) when the dispersible tablets were orally administered as water dispersion. In conclusion, the pharmacokinetics of four preparations of arbidol hydrochloride were not significant different in beagle dogs. However, compared with direct feeding, the absorption of arbidol hydrochloride was faster and the bioavailability was better when the dispersible tablets were orally administered as water dispersion.
References:
[1] JING-ZE LU. Pharmacokinetic comparison of four arbidol hydrochloride preparations in beagle dogs[J]. Biomedical Chromatography, 2021, 36 1. DOI:10.1002/bmc.5245.
You may like
Related articles And Qustion
See also
Lastest Price from Arbidol hydrochloride manufacturers
US $0.00/kg2025-11-25
- CAS:
- 131707-23-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/Kg/Bag2025-04-21
- CAS:
- 131707-23-8
- Min. Order:
- 1KG
- Purity:
- 99.0%~101.0%
- Supply Ability:
- 2000kg/month