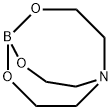
A substance with broad industrial uses: Triethanolamine borate
Description
Triethanolamine borate (TEOAB) is an environmentally friendly and cheap commercial material that can be easily prepared in one step by reacting boric acid and triethanolamine in an aqueous solution. This compound contains borate (-B-O-) and ethanolamine (-O-CH2-CH2-N-) groups. At present, TEOAB is widely used as a green rust inhibitor, lubricating additive, and flame retardant.

Synthetic method
The method for preparing triethanolamine borate comprises esterifying boric acid and .triethanolamine by heating and reacting the same under vacuum for periods of about 10 hours followed by recrystallization from acetonitrile or sublimation under vacuum and then by still further recrystallizations from pyridine.
Uses
Triethanolamine borate is of interest in industry because of its demonstrated effectiveness as a curing or hardening agent for glycidyl polyethers, also known as epoxy resins. When cured with triethanolamine borate, such resins have excellent chemical stability, high resistance to moisture permeability, low electrical losses, and superior adhesive qualities, all of which render the same particularly useful as surface coating materials, casting resins, and adhesive compounds.
Triethanolamine borate (TEOAB) possessing Lewis pair is an environmentally friendly, non-toxic, cheap, and water-soluble catalyst for the cycloaddition of CO2 with epoxides under solvent-free conditions. TEOAB contains one Lewis pair (boron as Lewis acid, tertiary amine as Lewis base), Lewis acid could activate the epoxide, and Lewis base could open the epoxide ring. Research has found that TEOAB is an efficient catalyst for converting CO2 into cyclic carbonates. With the assistance of tetrabutyl ammonium bromide (TBAB), the catalytic activity of TEOAB is further enhanced[1]. The quantitative yields of terminal cyclic carbonates can be obtained. Because of its excellent water solubility, TEOAB can be easily removed from water-insoluble cyclic carbonates with little loss by water washing to obtain purified products.
Triethanolamine borate could be a surface-stabilizing bifunctional Ni‐rich layered oxide cathode additive. The borate group provides effective CEI because the electrochemical decomposition of the borate functional group can generate uniform CEI layers on the cathode. Those layers prevent electron transfer while permitting Li+ migration at the interface, thereby improving the cycling retention of high-Ni NCM cathode[2]. The ethanolamine group, in turn, increases the interface stability of the Ni-rich NCM cathode because the ethanolamine group readily traps acidic H+ (or Li+) species by a chemical acid-base reaction to increase the binding properties between B and F− species in the cell. Therefore, using the bifunctional TEAB additive in the cell results in synergistic improvements by controlling surface reactivity by suppressing electrolyte decomposition with Ni dissolution.
References
[1] Yuansheng Ge, Hanzhong Ke, Guoe Cheng. “Triethanolamine borate as bifunctional Lewis pair catalyst for the cycloaddition of CO2 with epoxides.” Journal of CO2 Utilization 57 (2022): Article 101873.
[2] Sang Hoo Lim. “Triethanolamine borate as a surface stabilizing bifunctional additive for Ni-rich layered oxide cathode.” International Journal of Energy Research 45 2 (2020): 2138–2147.
You may like
See also
Lastest Price from Triethanolamine borate manufacturers

US $0.00-0.00/kg2025-05-08
- CAS:
- 283-56-7
- Min. Order:
- 1kg
- Purity:
- 95.1%
- Supply Ability:
- 1000kg

US $0.00/G/KG2025-04-22
- CAS:
- 283-56-7
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 1000MT