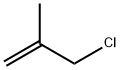
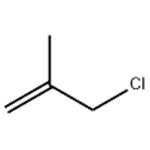
3-Chloro-2-methylpropene is used as an intermediate to produce carbofuran, herbicides, plastics, pharmaceuticals and other organics. It is also used as an additive in textile and perfumes. It was used to study ring opening polymerization of oxirane derivatives using organotin phosphate condensate and cationic polymerization of 3-Chloro-2-methylpropene with AICI3 and AIBr3 as initiators. It is also the reactant for the synthesis of cyclobutanone.
3-Chloro-2-methyl-1-propene was used to study ring opening polymerization of oxirane derivatives using organotin phosphate condensate. It was used to study cationic polymerization of 3-Chloro-2-methyl-1-propene using using AICI3 and A3IBr, as initiators.
3-Chloro-2-methylpropene has been used as reactant in the synthesis of cyclobutanone. It is used as an intermediate in the production of plastics, pharmaceuticals and other organic chemicals.
ChEBI: 3-Chloro-2-methylpropene is an organochlorine compound.
MAC is a valuable index for clinical anesthesia, but it is
seldom employed without taking other factors into consideration.
For example, inhibiting movement in only
50% of patients is not acceptable. Consequently, if an
inhalational agent were being used alone—that is, without
the administration of other anesthetics or analgesic
drugs—the anesthesiologist would employ a multiple of
its MAC value to ensure immobility.MAC is frequently
multiplied by a factor of 1.3 to achieve nearly 100%
clinical efficacy. On the other hand, useful clinical results
may be achieved with doses of anesthetics below
MAC levels. For example, mild analgesia and amnesia
often occur with doses of inhalational agents that are
near 0.5 MAC. In this state, it may even be possible to
communicate with patients intraoperatively, while their
recall is limited.
A colorless to straw-colored liquid with a sharp penetrating odor. Less dense than water and insoluble in water. Flash point below 0°F. May be toxic by ingestion. Irritating to skin and eyes. Used to make plastics and pharmaceuticals.
Highly flammable. 3-Chloro-2-methylpropene may react with water at elevated temperatures. . Insoluble in water.
3-Chloro-2-methylpropene is sensitive to light. 3-Chloro-2-methylpropene can react vigorously with oxidizers. 3-Chloro-2-methylpropene is incompatible with strong bases. 3-Chloro-2-methylpropene may react with water at elevated temperatures. .
Irritant; poison; anesthetic; questionable
carcinogen; toxic.
Inhalation causes irritation of nose and throat. Contact of vapor or liquid with eyes causes irritation. Liquid irritates skin. Ingestion causes irritation of mouth and stomach.
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, and tumorigenic data.
Experimental reproductive effects. An
irritant. Human mutation data reported.
Very dangerous fire hazard when exposed to
heat, flame, or oxidizers. Moderately
explosive when exposed to heat or flame.
Can react vigorously with oxidizing
materials. To fight fire, use alcohol foam,
CO2, dry chemical. When heated to
decomposition it emits toxic fumes of Cl-.
See also CHLORINATED
HYDROCARBONS, ALIPHATIC; and
ALLYL COMPOUNDS.
3-Chloro-2-methylpropene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
The initial risk screening level (IRSL) is 0.03μg/m3 with an annual averaging time.