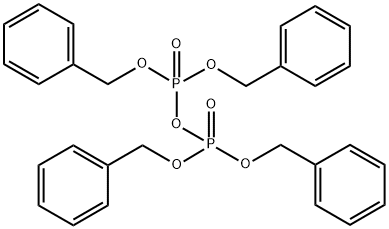
Tetrabenzyl pyrophosphate and diphenylphosphinic anhydride, with two phosphoryl groups (P O) as ligating sites, can be used as novel ionophores to make Pb2+-selective membrane electrodes. A good result was obtained with tetrabenzyl pyrophosphate, and the electrode based on this ionophore and bis(1-butylpentyl) adipate as a solvent mediator in a poly(vinyl chloride) membrane matrix exhibited a near-Nernstian response to Pb2+ in the concentration range of 1×10?5–1×10?2 M with a slope of 28.7 mV per concentration decade in a solution containing 0.1 M Mg(NO3)2. Tetrabenzyl pyrophosphate showed the best response to Pb2+, almost free from interference by other metal cations. By adding the ionic additive potassium tetrakis(p-chlorophenyl)borate (KTpClPB; 40 mol% relative to tetrabenzyl pyrophosphate), a drastic change occurred in the electrode response, showing a monovalent species probably due to the formation of PbA+, where A stands for anions present in the sample solution, and decreased the selectivity of the electrode to other metal cations[1].
Tetrabenzyl pyrophosphate is used in the preparation of phosphoryl derivatives of shikimic acid in the presence of LDA. It is used in the preparation of dibenzyl phosphoro fluoridate in the presence of cesium fluoride as a catalyst. It is also used as a precursor in pharmaceuticals and involved in the phosphorylation of inositol derivatives.
The production of tetrabenzyl pyrophosphate by the action of acyl chlorides on dibenzyl hydrogen phosphate, and a novel reaction of tetraphenyl pyrophosphate.
Preparation of Tetrabenzyl Pyrophosphate: A flask fitted with a nitrogen inlet, overhead stirrer, teflon-coated thermocouple probe, and pressure-equalizing addition funnel was charged with 350 milliliters of dry (water content ≦50 μg/mL), peroxide-free tetrahydrofuran, followed by 50.0 grams (174 millimoles) of dibenzylphosphoric acid (DBP), and the resulting mixture was stirred until the solid dissolved (about 10-15 minutes). A solution of 18.9 grams (91.6 millimoles) of dicyclohexylcarbodiimide (DCC) in 215 milliliters of THF was added from the addition funnel to a stirred, cooled (water-bath) solution of DBP at a rate to maintain the temperature at about 20°-25° C. The reaction is slightly exothermic and the addition took about 30 minutes. Within minutes, a precipitate of dicyclohexylurea formed in the mixture. Stirring was continued for about 2 hours at 20°-25° C. The reaction was monitored by HPLC assay using VYDAC C-18 (300A, 4.6×250 mm) column with water (0.02M KH2PO4) acetonitrile as eluent. The reaction was complete in about 1 hour (assay showing <2 percent unreacted DBP). The mixture was then filtered while excluding moisture to remove dicyclohexylurea. The filter cake was washed with two 25 milliliter portions of THF. The solution when assayed by HPLC showed 45.9 grams (98 percent) yield of tetrabenzyl pyrophosphate (TBPP) in 620 milliliters of THF (0.137M). The filtrate was then stored at 0° C. with exclusion of moisture until the next step.
[1] Dafeng Xu, Takashi Katsu. “Tetrabenzyl pyrophosphate as a new class of neutral carrier responsive to lead ion.” Talanta 51 2 (2000): Pages 365-371.