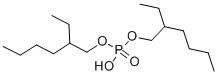
diisoctyl acid phosphate is a colorless liquid. diisoctyl acid phosphate will burn though diisoctyl acid phosphate may require some effort to ignite. diisoctyl acid phosphate is corrosive to metals and tissue.
diisoctyl acid phosphate is an organophosphate ester, and behaves as an acid. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Moderately toxic by
irritation to skin, eyes, and mucous
membranes. A corrosive compound. When
heated to decomposition it emits toxic
fumes of POx. See also PHOSPHATES.
Contaminants of commercial samples include the monoester, polyphosphates, pyrophosphate, 2-ethylhexanol and metal impurities. Dissolve the acid in n-hexane to give an 0.8M solution. Wash this with an equal volume of M HNO3, then with saturated (NH4)2CO3 solution, with 3M HNO3, and twice with water [Petrow & Allen Anal Chem 33 1303 1961]. Similarly, the impure sodium salt, after scrubbing with pet ether, is acidified with HCl and the free organic acid is extracted into pet ether and purified as above [Peppard et al. J Inorg Nucl Chem 7 231 1958], or as described by Stewart & Crandall [J Am Chem Soc 73 1377 1951]. It can be purified via its copper salt [McDowell et al. J Inorg Nucl Chem 38 2127 1976]. [Beilstein 1 IV 1796.]