m-Phenylenediamine is a colorless to white crystalline substance that turns red upon exposure to air
The appearance of the high purity of the m-phenylenediamine is snow white flake solid used as a synthetic electronic grade polyimide material and epoxy resin curing agent.
1,3-Phenylenediamine is used in the foaming-type hair dye composition.
m-Phenylenediamine is used in the foaming-type hair dye composition. It is used as a synthetic electronic grade polyimide material and epoxy resin curing agent. It is used also block polymers, textile fibers, urethanes, petroleum additives, rubber chemicals, in corrosion inhibitors, in photography, as reagent for gold & bromine.
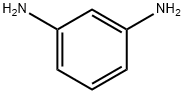
ChEBI: 1,3-phenylenediamine is a phenylenediamine taht is benzene substituted at positions 1 and 3 with amino functions.
Colorless or white colored needles that turn red or purple in air. Melting point 64-66 C. Density 1.14 g / cm3. Flash point 280 F. May irritate skin and eyes. Toxic by skin absorption, inhalation or ingestion. Used in aramid fiber manufacture, as a polymer additive, dye manufacturing, as a laboratory reagent, and in photography.
Soluble in water [Merck].
m-Phenylenediamine an aromatic amine, neutralizes acids, acid chlorides, acid anhydrides and chloroformates in exothermic reactions to form salts. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Incompatible with oxidizing agents .
m-Phenylenediamine is combustible. Dust may form explosive mixtures in air
Flammability and Explosibility
Non flammable
Suspected carcinogen with experimental tumorigenic and teratogenic data. Poison by ingestion, intravenous, subcutaneous, and intraperitoneal routes. Mildly toxic by skin contact. Mutation data reported. Combustible when exposed to heat or flame. A hair dye ingredtent. When heated to decomposition it emits toxic fumes of NOx. See also other phenylenediamine entries and AMINES.
Used in making various dyes; as a curing agent for epoxy resin; rubber, textile fibers; urethanes, corrosion inhibitors; adhesives; in photographic and analytical procedures and processes.
"Occupational exposure to m-PDA may occur through
inhalation and dermal contact with this compound at workplace
where m-PDA is produced or used. The general population may be exposed to m-PDA via dermal contact
with consumer products containing this compound."An IARC Working
Group concluded, on the basis of lack of human data and
inadequate animal data, that m-PDA was not classifiable
(Group 3) as to its carcinogenicity to humans.
By the perfused rat liver, 1,3-diaminobenzene (MPD)
is metabolized to three identified N-acetylated
derivatives N-acetyl-1,3-diaminobenzene, N,N'-
diacetyl-1,3-diaminobenzene, and N,N'-diacetyl-2,4-
diaminophenol which are identical to the metabolites
excreted in rat urine.
UN1673 Phenylenediamines (o-, m-, p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purify the diamine by distillation under a vacuum followed by recrystallisation from EtOH (rhombs) and if necessary redistillation. It should be protected from light; otherwise it darkens rapidly. [Neilson et al. J Chem Soc 371 1962, IR: Katritzky & Jones J Chem Soc 3674, 2058 1959, UV: Forbes & Leckie Can J Chem 36 1371 1958.] The hydrochloride has m 277-278o, and the bis-4-chlorobenzenesulfonyl derivative has m 220-221o from H2O (214-215o, from MeOH/H2O) [Runge & Pfeiffer Chem Ber 90 1737 1957]. The acetate has m 191o. [Beilstein 13 IV 79.]
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides; chloroformates. Heat and light contribute to instability. Keep away from metals.
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device.