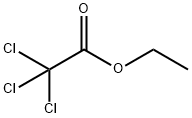
Colorless liquid with a menthol-like odor. It is miscible with ethanol, ether and benzene, but insoluble in water.
Ethyl trichloroacetate was used in the synthesis of dichlorocarbon precursor, phenyl(trichloromethyl)methyl.
Ethyl trichloroacetate can be used as solvent, organic synthesis, perfume and pharmaceutical intermediates.
Ethyl trichloroacetate is synthesized from trichloroacetic acid and ethyl alcohol by the esterification reaction.
synthesis steps: Trichloroacetic acid(TCA), anhydrous ethyl alcohol and sulfuric acid are heated together and refluxed for 6 h. After cooling, the ester layer is separated by pouring into water, neutralized with 5-10% sodium carbonate solution, washed with water and dried overnight with anhydrous calcium chloride. After filtration, distillation was carried out to obtain the finished product. Yield 75%.
Ethyl trichloroacetate undergoes atom-transfer radical addition reaction with styrene catalyzed by the Ru-pentamethylcyclopentadienyl complexes.
Shake the ester with saturated aqueous Na2CO3 (three times), aqueous 50% CaCl2 (three times), saturated aqueous NaCl (twice), then distil over CaCl2 and redistil it under reduced pressure. [Beilstein 2 IV 514.]