clear colorless to light yellow viscous liquid
DL-Alaninol has been used in the synthesis of N6-α-(I-hydroxypropyl) lysine, diastereomers of DL-β-amino alcohols, enantiopure and racemic samples of 2-methyl-N-tosylaziridine, (±)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide.
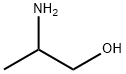
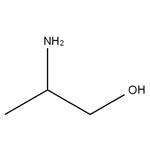
A colorless to pale yellow liquid with a fishy odor. Moderately toxic by ingestion and skin contact. A severe skin irritant. Combustible. The compound contains both the amine group -NH2 and the alcohol group -OH and so has some properties of both. Floats and mixes with water.
DL-Alaninol is a chemical base. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
If inhaled may be harmful. Contact may cause burns to skin and eyes. (Organic base.)
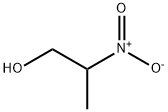
The general procedure for the synthesis of DL-aminopropanol from 2-nitro-1-propanol was carried out as follows: to an acetonitrile solution of 2-nitro-1-propanol (1 mmol / eq) was added DIPEA (5 mmol / eq) at 15 °C, stirring was maintained. Subsequently, freshly distilled trichlorosilane (3.5 mmol / eq) was added slowly dropwise using a syringe. The reaction mixture was stirred continuously at 15°C for 18 hours. Upon completion of the reaction, the reaction was quenched by addition of 10% NaOH solution and the mixture was extracted with ethyl acetate (AcOEt). The organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated in vacuum to give DL-aminopropanol in quantitative yield.
[1] Patent: WO2014/37444, 2014, A1. Location in patent: Page/Page column 6
[2] Organic Letters, 2015, vol. 17, # 16, p. 3941 - 3943
[3] Journal of the American Chemical Society, 1945, vol. 67, p. 206
[4] Journal of the Chemical Society, 1949, p. 510,515
[5] Acta Chemica Scandinavica, 1976, vol. 30, # 6 B, p. 567 - 573