To a round-bottomed flask was added 2.5 L of water followed by 80 g of sodium hydroxide and stirred until completely dissolved. Next, 500 g of guaiacol and 1.12 kg of 1-chloro-2,3-epoxypropane were added and the reaction was continuously stirred at 25-35°C for 5-6 hours. After completion of the reaction, the organic layer was separated. To the separated obtained epichlorohydrin layer was added 160 g of sodium hydroxide dissolved in 2.5 L of water and stirring was continued at 25-30 °C for 3-4 hours. The organic layer was again separated and the organic layer was washed with 150 g of sodium hydroxide dissolved in 1.5 L of water. Subsequently, the product layer was distilled at 90°C and 600-700 mmHg under vacuum to recover excess epichlorohydrin, resulting in 650-680 g of oily product. To this crude product was added 3.0 L of isopropanol, cooled to 0 °C and filtered to give 1-(2-methoxyphenoxy)-2,3-epoxypropane (3). Yield: 80%; purity >98%.
[1] Organic Process Research and Development, 2012, vol. 16, # 10, p. 1660 - 1664
[2] Medicinal Chemistry Research, 2004, vol. 13, # 8-9, p. 631 - 642
[3] Synthetic Communications, 1994, vol. 24, # 6, p. 833 - 838
[4] Tetrahedron, 2006, vol. 62, # 47, p. 10968 - 10979
[5] Angewandte Chemie - International Edition, 2014, vol. 53, # 26, p. 6641 - 6644
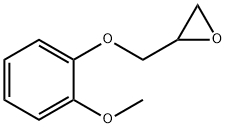


![[(2-Methoxyphenoxy)Methyl]Oxirane pictures](/ProductImageEN2/2025-10/Small/433abb1b-8d4d-4c5b-a706-6c200f140807.png)
