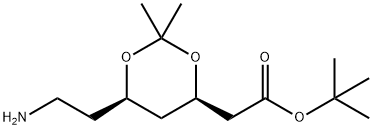
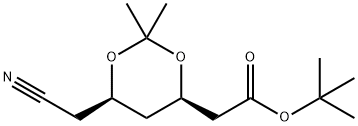
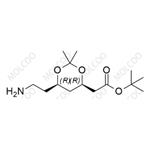
General procedure for the synthesis of the target compound (CAS:125971-86-0) from (4R-cis)-6-cyanomethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester: to a 5-gallon stainless steel reactor were added 250 g of Raney nickel (Ra-Ni), (4R,6R)-6-cyanomethyl-2,2-dimethyl-[1,3] tert-butyl dioxane-4-yl-acetate (1.0 kg, 3.71 mol), toluene (6 L), methanol (675 mL) and 6.5 M ammonia/methanol solution (800 mL). The reactor was sealed and pressure tested with nitrogen (N2) to 3.5 bar and purged three times with N2 at 3.5 bar. Subsequently, the reactor was purged with hydrogen (H2) three times to 3.5 bar, without turning on stirring during this process. After H2 pressurization to 3.5 bar, stirring was initiated and the reaction was carried out for 2-6 h. A slight exotherm was observed and the temperature increased to 30-40 °C. Stirring was continued until H2 absorption ceased, then stirring was continued at 30 to 40 °C for 30 min. The reaction mixture was cooled to 20-25 °C, the H2 source and stirrer were turned off, and the reactor was drained of H2. The stirrer was restarted, and the reactor was purged with N2 three times to 3.5 bar. The recovered nickel catalyst was filtered under nitrogen protection and the reactor and catalyst bed were washed with toluene (250 mL). The filtrates were combined and concentrated under reduced pressure to about 500 mL at no more than 55°C. [Note: the vacuum was broken with nitrogen after the concentration was completed]. Add saturated sodium chloride solution to the concentrate and stir under nitrogen protection for 10 minutes. Stop stirring and let stand to stratify. The lower aqueous phase was discarded and the organic phase was concentrated to give a yellow oily target product (1.054 kg, 104% yield, containing 7% residual toluene); 1H-NMR (400 MHz, CDCl3): δ 4.23-4.19 (m, 1H), 3.99-3.95 (m, 1H), 2.74 (t, J=7.1 Hz, 2H), 2.40-2.36 (m, 1H), 2.27-2.22 (m, 1H), 1.58-1.41 (m, 2H), 1.40 (s, 9H), 1.31 (s, 6H), 0.89 (s, 9H); low-resolution mass spectra (APCI) m/z 273 [M+H]+.