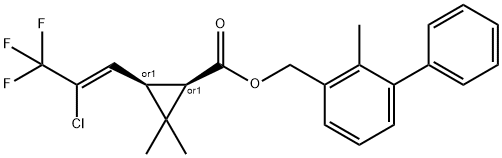
Bifenthrin is synthetic pyrethroid insecticide with biochemical origins in the natural insecticide pyrethrum. It is a waxy solid with a color ranging from an off-white to a pale tan, and with a slightly sweet odor. It is almost insoluble in water. Bifenthrin is used for the control of borers and termites in timber, insect pests in agricultural crops (bananas, apples, pears, ornamentals) and turf, as well as for general pest control (spiders, ants, fleas, flies, mosquitoes). Due to its high toxicity to aquatic organisms, it is listed as a restricted use pesticide. It has a very low solubility in water and tends to bind to soil, which minimizes runoff into water sources. It is marketed as Talstar, Bifenthrine, Brigade, Capture, Torant, Zipak, FMC 54800, and OMS3024.
[1] http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=DE&selectedID=1026
[2] http://apvma.gov.au/node/12396
[3] http://www.ehrf.info/wp-content/uploads/2012/03/Bifenthrin-Feb-2012.pdf
Bifenthrin is a synthetic pyrethroid insecticide/miticide/acaricide. Bifenthrin is off-white
to pale tan waxy solid granules with a faint, musty odour and a slightly sweet smell.
Bifenthrin is soluble in methylene chloride, acetone, chloroform, ether, and toluene and
slightly soluble in heptane and methanol. It is slightly combustible and support combustion
at elevated temperatures. Thermal decomposition and burning may form toxic byproducts
such as carbon monoxide, carbon dioxide, hydrogen chloride, and hydrogen
fluoride. Bifenthrin treatment affects the nervous system and causes paralysis in insects.
Bifenthrin is a member of the pyrethroid chemical class. It is an insecticide and acaricide.
Bifenthrin is off-white to pale tan waxy solid granules with a faint, musty odor and slightly
sweet smell. Bifenthrin is soluble in methylene chloride, acetone, chloroform, ether, and
toluene, and is slightly soluble in heptane and methanol. It is slightly combustible and support combustion at elevated temperatures. Thermal decomposition and burning may form
toxic by-products, such as carbon monoxide, carbon dioxide, hydrogen chloride, and hydrogen fl uoride. Bifentrin treatment affects the nervous system and causes paralysis in insects.
Off white to pale tan waxy substance. Faint,
sweet odor.
Bifenthrin controls foliar insect pests and some mites on a range of
crops including cereals, citrus, cotton, fruit and vegetables.
Third generation synthetic pyrethroid. Insecticide, acaricide.
ChEBI: A carboxylic ester obtained by formal condensation of cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic acid and [(2-methyl-1,1'-biphenyl)-3-yl]methanol.
Bifenthrin is moderately toxic to species of mammals when ingested. Exposures to large doses
of bifenthrin cause poisoning with symptoms that include, but are not limited to, incoordination, tremor, salivation, vomiting, diarrhea, and irritability to sound and touch. Exposures to bifenthrin through skin absorption and/or inhalation of dust cause adverse health effects.
On contact with bifenthrin, occupational workers develop adverse health effects that include
skin sensations, rashes, numbness, and a burning and tingling type of effect. As a pyrethroid
poison, bifenthrin disturbs the electrical impulses in nerves, over-stimulating nerve cells, causing tremors and eventually causing paralysis. The skin-related health effects were found to be
reversible and subside after a brief period of time and stoppage of further exposures to bifenthrin. Although bifenthrin causes no infl ammation or irritation on human skin, it can cause a
tingling sensation that lasts about 12 h. Bifenthrin has caused no symptoms of irritation to rabbits’ eyes. The US EPA has classifi ed bifenthrin as toxicity class II, meaning moderately toxic
Insecticide, Acaricide: A broad-spectrum insecticide. Registered to control
cone worms, seed bugs, seed worms and other insects and
mites on rangeland, forests and right-of-ways. It is also
used to control household and lawn pests. A U.S. EPA restricted
Use Pesticide (RUP).
BIFLEX®; BISTAR®; BRIGADE®;
CAPTURE® Bifenthrin; DISCIPLINE®; DOUBLE
THREAT®; EMPOWER®; FMC® 54800; FMC® 58000;
TALSTAR®; TALSTAR LAWN & TREE®; TORANT®;
ZIPAK®
A broad spectrum pyrethroid secticide/
acaricide used to control cone worms, seed bugs, seed
worms and other insects and mites in forests, on rangeland,
and right-of-ways. It is also used to control household and
lawn pests. A United States Environmental Protection
Agency Restricted Use Pesticide (RUP).
When mites are administered 14C-bifenthrin either by
injection or by contact, bifenthrin is efficiently
metabolized by the mites and the metabolites
identified arise from the combination of ester cleavage,
oxidation, and conjugation reactions and are the
4'-hydroxy derivative of the ester, the primary ester
cleavage products, the acid, and its 4'-hydroxy
derivative from the alcohol moiety, as well as several
unidentified metabolites.
Bifenthrin should be kept stored in a cool, dry, well-ventilated place away from heat, open
flame or hot surfaces. It should only be stored in its original containers and should not be
contaminated with other pesticides, fertilizers, water, food, or feed by storage or disposal
UN2588 Pesticides, solid, toxic, Hazard Class:
6.1; Labels: 6.1—Poisonous materials, Technical Name
Required. UN3349 Pyrethroid pesticide, solid toxic, Hazard
Class: 6.1; Labels: 6.1—Poisonous material.
The
solubility of bifenthrin in water is relatively low at 0.1 mg l-1.
Furthermore, the water octanol coefficient (Kow) is 1×106 and
results in bifenthrin binding to organic substrates. The soil
sorption coefficients range between 1.31�×105 and 3.02�×105,
indicating relatively tight binding to soil particles. They also tend
to bind to organic particulate materials in the water column. The
Kow of bifenthrin may explain the bioconcentration factors
(BCFs) observed in some animals, especially fish. For example,
fathead minnows (Pimephales promelas) exposed to 0.0037 mg l�1
bifenthrin had BCFs of 21 000 after 127 days and 28 000 after
254 days of exposure. Hydrolysis of bifenthrin in buffered water
occurs between pH 5 and 9. Photolysis studies in water and soil
found that the half-lives were 408 and 96.9 days, respectively.
The aerobic soil half-life was 96.3 days and the anaerobic halflife,
425 days.
Bifenthrin is stable for 21 days in the pH range 5-9 at 21°C but it is labile
at higher pH. It has a DT50 of 225 days in natural daylight and can therefore
be regarded as relatively photochemically stable (PSD).
May react violently with strong oxidizers,
bromine, 90% hydrogen peroxide, phosphorus
trichloride, silver powders or dust. Incompatible with silver
compounds, lime, and ordinary soaps. Mixture with some
silver compounds forms explosive salts of silver oxalate.
Incineration would be an
effective disposal procedure where permitted. If an efficient
incinerator is not available, the product should be mixed
with large amounts of combustible material and contact
with the smoke should be avoided. In accordance with
40CFR165, follow recommendations for the disposal of
pesticides and pesticide containers.